Combination of Pelargonium sidoides and Coptis chinensis root inhibits nuclear factor kappa B-mediated inflammatory response in vitro and in vivo
- PMID: 29351747
- PMCID: PMC5775528
- DOI: 10.1186/s12906-018-2088-x
Combination of Pelargonium sidoides and Coptis chinensis root inhibits nuclear factor kappa B-mediated inflammatory response in vitro and in vivo
Abstract
Background: Pelargonium sidoides (PS) and Coptis chinensis root (CR) have traditionally been used to treat various diseases, including respiratory and gastrointestinal infections, dysmenorrhea, and hepatic disorders. The present study was conducted to evaluate the anti-inflammatory effects of a combination of PS and CR in vitro and in vivo.
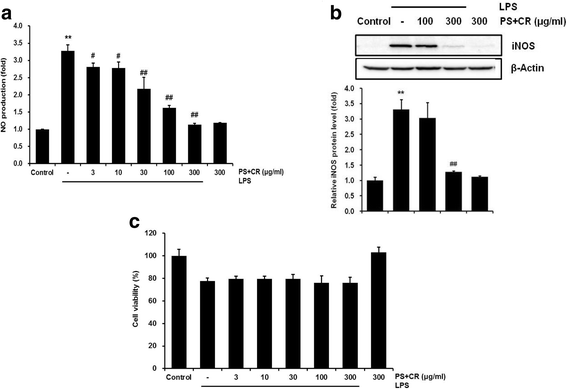
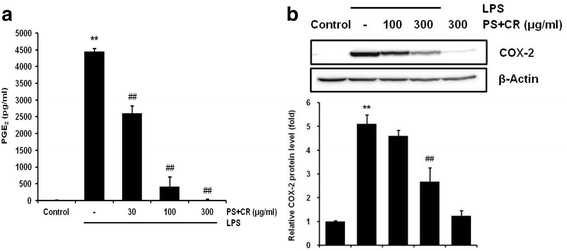
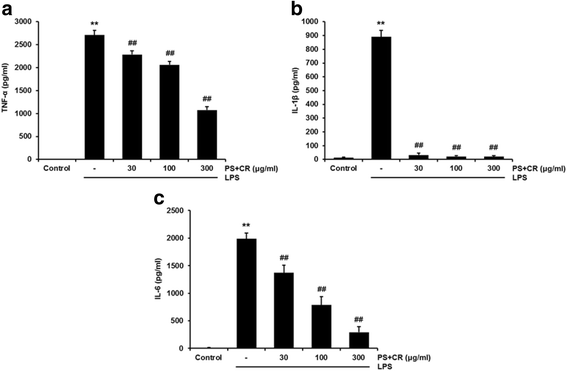
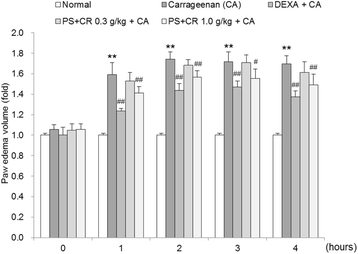
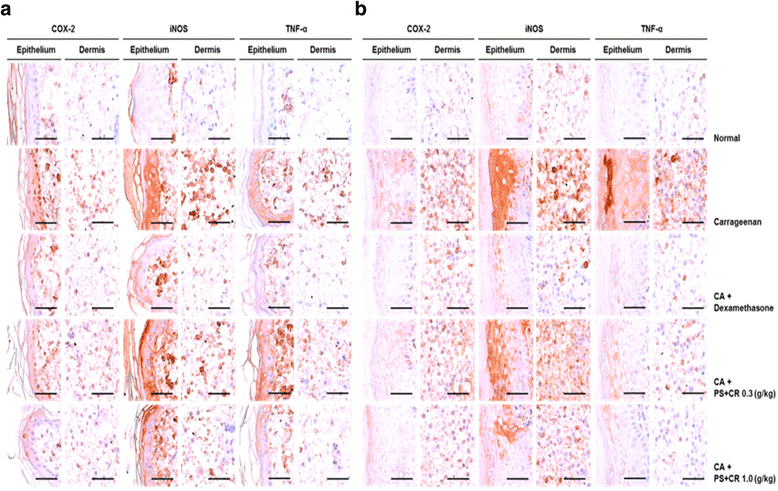
Methods: The in vitro effects of PS + CR on the induction of inflammation-related proteins were evaluated in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells. The levels of nitric oxide (NO) and of inflammatory cytokines and prostaglandin E2 (PGE2) were measured using the Griess reagent and enzyme-linked immunosorbent assay (ELISA) methods, respectively. The expression of inflammation-related proteins was confirmed by Western blot. Additionally, the effects of PS + CR on paw edema volume, skin thickness, and numbers of infiltrated inflammatory cells, mast cells, COX-2-, iNOS-, and TNF-α-immunoreactive cells in dorsum and ventrum pedis skin were evaluated in a rat model of carrageenan (CA)-induced paw edema.
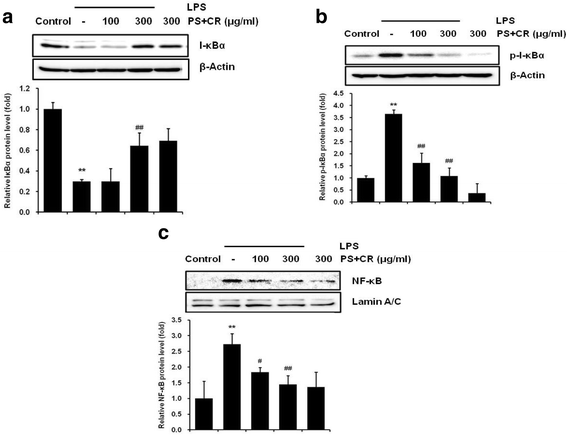
Results: PS + CR significantly reduced production of NO, PGE2 and three pro-inflammatory cytokines (tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, and IL-6) and also decreased levels of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2). Treatment with PS + CR significantly reduced the protein expression levels of LPS-stimulated nuclear factor kappa B (NF-κB) and phosphorylated inhibitor of NF-κB (p-I-κBα). Additionally, PS + CR significantly inhibited the increases in paw swelling, skin thickness, infiltrated inflammatory cells, mast cell degranulation, COX-2-, iNOS-, and TNF-α-immunoreactive cells in the rat model of CA-induced acute edematous paw.
Conclusions: These results demonstrate that PS + CR exhibits anti-inflammatory properties through decreasing the production of pro-inflammatory mediators (NO, PGE2, TNF-α, IL-1β, and IL-6), suppressing NF-κB signaling in LPS-induced RAW 264.7 cells. Additionally, the results of the CA-induced rat paw edema assay revealed an anti-edema effect of PS + CR. Furthermore, it is suggested that PS + CR also inhibits acute edematous inflammation by suppressing mast cell degranulation and inflammatory mediators (COX-2, iNOS, and TNF-α). Thus, PS + CR may be a potential candidate for the treatment of various inflammatory diseases, and it may also contribute to a better understanding of the molecular mechanisms underlying inflammatory response regulation.
Keywords: Coptis chinensis root; Histopathology; Inflammation; Nuclear factor kappa B; Paw edema; Pelargonium sidoides.
Conflict of interest statement
Ethics approval
All animal experiments were approved by the Animal Ethical Care Committee of Daegu Haany University (Approval number: DHU2016–089) and conducted in accordance with the guidelines for the care and use of laboratory animals at Daegu Haany University.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures

References
-
- Jang KJ, Choi SH, Yu GJ, Hong SH, Chung YH, Kim CH, Yoon HM, Kim GY, Kim BW, Choi YH. Anti-inflammatory potential of total saponins derived from the roots of Panax Ginseng in lipopolysaccharide-activated RAW 264.7 macrophages. Exp Ther Med. 2016;11:1109–1115. doi: 10.3892/etm.2015.2965. - DOI - PMC - PubMed
-
- Kim SY, Park SM, Hwangbo M, Lee JR, Byun SH, Ku SK, Cho IJ, Kim SC, Jee SY, Park SJ. Cheongsangbangpung-tang ameliorated the acute inflammatory response via the inhibition of NF-kappaB activation and MAPK phosphorylation. BMC Complement Altern Med. 2017;17:46. doi: 10.1186/s12906-016-1501-6. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

