The Jun/miR-22/HuR regulatory axis contributes to tumourigenesis in colorectal cancer
- PMID: 29351796
- PMCID: PMC5775639
- DOI: 10.1186/s12943-017-0751-3
The Jun/miR-22/HuR regulatory axis contributes to tumourigenesis in colorectal cancer
Abstract
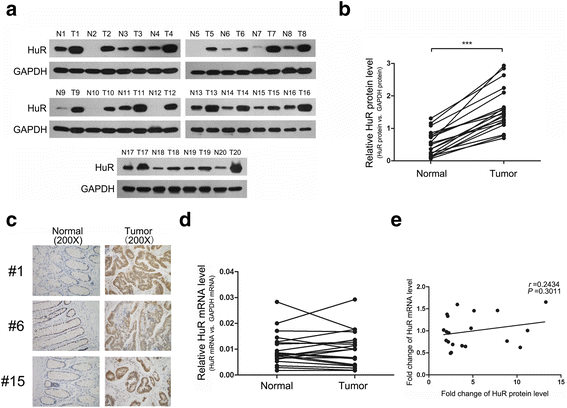
Background: Colorectal cancer (CRC) is a severe health problem worldwide. Clarifying the mechanisms for the deregulation of oncogenes and tumour suppressors in CRC is vital for its diagnosis, treatment, prognosis and prevention. Hu antigen R (HuR), which is highly upregulated in CRC, functions as a pivotal oncogene to promote CRC progression. However, the underlying cause of its dysregulation is poorly understood.
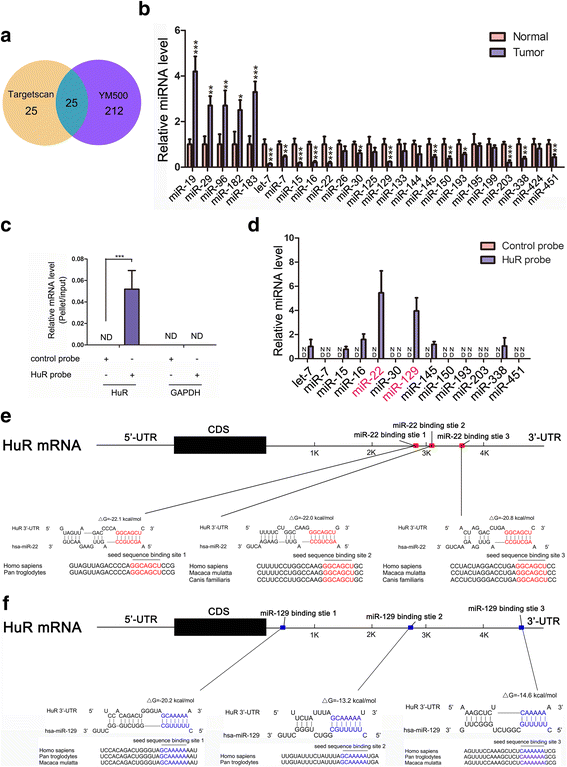
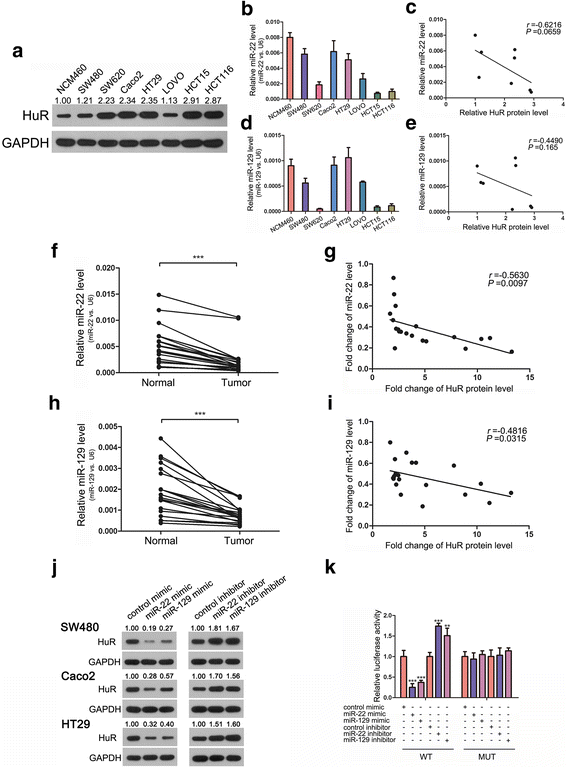
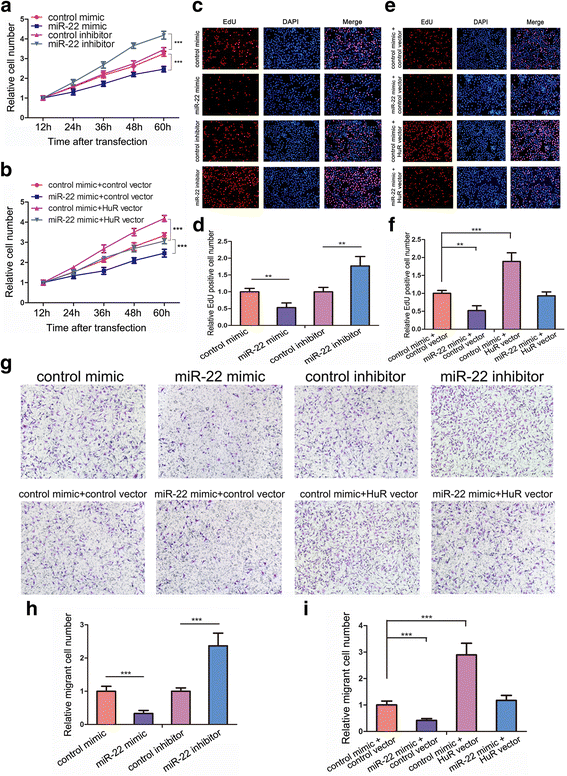
Methods: In CRC tissue sample pairs, HuR protein levels were measured by Western blot and immunohistochemical (IHC) staining, respectively. HuR mRNA levels were also monitored by qRT-PCR. Combining meta-analysis and microRNA (miRNA) target prediction software, we predicted miRNAs that targeted HuR. Pull-down assay, Western blot and luciferase assay were utilized to demonstrate the direct binding of miR-22 on HuR's 3'-UTR. The biological effects of HuR and miR-22 were investigated both in vitro by CCK-8, EdU and Transwell assays and in vivo by a xenograft mice model. JASPAR and SABiosciences were used to predict transcriptional factors that could affect miR-22. Luciferase assay was used to explore the validity of putative Jun binding sites for miR-22 regulation. ChIP assay was performed to test the Jun's occupancy on the C17orf91 promoter.
Results: We observed a significant upregulation of HuR in CRC tissue pairs and confirmed the oncogenic function of HuR both in vitro and in vivo. We found that an important tumour-suppressive miRNA, miR-22, was significantly downregulated in CRC tissues and inversely correlated with HuR in both CRC tissues and CRC cell lines. We demonstrated that miR-22 directly bound to the 3'-UTR of HuR and led to inhibition of HuR protein, which repressed CRC proliferation and migration in vitro and decelerated CRC xenografted tumour growth in vivo. Furthermore, we found that the onco-transcription factor Jun could inhibit the transcription of miR-22.
Conclusions: Our findings highlight the critical roles of the Jun/miR-22/HuR regulatory axis in CRC progression and may provide attractive potential targets for CRC prevention and treatment.
Keywords: Colorectal cancer; HuR; Jun; Migration; Proliferation; miR-22.
Conflict of interest statement
Ethics approval
CRC tissues were collected from patients who underwent surgical resection at the Affiliated Drum Tower Hospital of Nanjing University Medical School (Nanjing, China). All patients signed consent letters and all manipulation of the tissues was approved by the Ethics Committee of Nanjing University. All experiments were performed in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) and the guidelines of the Nanjing University.
All animal experiments complied with the ARRIVE guidelines and were carried out in accordance with the National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) and the guidelines of the Nanjing University.
Consent for publication
We have obtained consents to publish this paper from all the participants of this study.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
Similar articles
-
miR-19a promotes colorectal cancer proliferation and migration by targeting TIA1.Mol Cancer. 2017 Mar 4;16(1):53. doi: 10.1186/s12943-017-0625-8. Mol Cancer. 2017. PMID: 28257633 Free PMC article.
-
Exploiting a novel miR-519c-HuR-ABCG2 regulatory pathway to overcome chemoresistance in colorectal cancer.Exp Cell Res. 2015 Nov 1;338(2):222-31. doi: 10.1016/j.yexcr.2015.09.011. Epub 2015 Sep 18. Exp Cell Res. 2015. PMID: 26386386
-
Transcription factor SP1-induced microRNA-146b-3p facilitates the progression and metastasis of colorectal cancer via regulating FAM107A.Life Sci. 2021 Jul 15;277:119398. doi: 10.1016/j.lfs.2021.119398. Epub 2021 Apr 5. Life Sci. 2021. PMID: 33831429
-
The Dual Role of MicroRNAs in Colorectal Cancer Progression.Int J Mol Sci. 2018 Sep 17;19(9):2791. doi: 10.3390/ijms19092791. Int J Mol Sci. 2018. PMID: 30227605 Free PMC article. Review.
-
Unraveling the Regulatory Role of HuR/microRNA Axis in Colorectal Cancer Tumorigenesis.Cancers (Basel). 2024 Sep 18;16(18):3183. doi: 10.3390/cancers16183183. Cancers (Basel). 2024. PMID: 39335155 Free PMC article. Review.
Cited by
-
Recent advances in the diagnostic and therapeutic roles of microRNAs in colorectal cancer progression and metastasis.Front Oncol. 2022 Oct 13;12:911856. doi: 10.3389/fonc.2022.911856. eCollection 2022. Front Oncol. 2022. PMID: 36313731 Free PMC article. Review.
-
Cervical squamous cell carcinoma-secreted exosomal miR-221-3p promotes lymphangiogenesis and lymphatic metastasis by targeting VASH1.Oncogene. 2019 Feb;38(8):1256-1268. doi: 10.1038/s41388-018-0511-x. Epub 2018 Sep 25. Oncogene. 2019. PMID: 30254211 Free PMC article.
-
SOX9/MKLN1-AS Axis Induces Hepatocellular Carcinoma Proliferation and Epithelial-Mesenchymal Transition.Biochem Genet. 2022 Dec;60(6):1914-1933. doi: 10.1007/s10528-022-10196-6. Epub 2022 Feb 9. Biochem Genet. 2022. PMID: 35138470
-
Derivation and Clinical Validation of a Redox-Driven Prognostic Signature for Colorectal Cancer.Front Oncol. 2021 Oct 27;11:743703. doi: 10.3389/fonc.2021.743703. eCollection 2021. Front Oncol. 2021. PMID: 34778061 Free PMC article.
-
5-FU blocks shuttling of HuR mediated by PKCδ in gastric cancer cells.Transl Cancer Res. 2020 Aug;9(8):4790-4799. doi: 10.21037/tcr-20-2129. Transl Cancer Res. 2020. PMID: 35117842 Free PMC article.
References
-
- Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- No. 2014CB542300/National Basic Research Program of China (973 Program)/International
- No. 31741075/National Natural Science Foundation of China/International
- No. BK20140601/Natural Science Foundation of Jiangsu Province/International
- No. BE2016737/Natural Science Foundation of Jiangsu Province/International
- No. 020814380070/Fundamental Research Funds for the Central Universities/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

