Targeting the SphK1/S1P/S1PR1 Axis That Links Obesity, Chronic Inflammation, and Breast Cancer Metastasis
- PMID: 29351902
- PMCID: PMC6945803
- DOI: 10.1158/0008-5472.CAN-17-1423
Targeting the SphK1/S1P/S1PR1 Axis That Links Obesity, Chronic Inflammation, and Breast Cancer Metastasis
Abstract
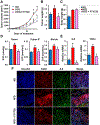
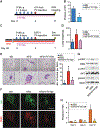
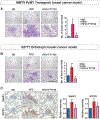
Although obesity with associated inflammation is now recognized as a risk factor for breast cancer and distant metastases, the functional basis for these connections remain poorly understood. Here, we show that in breast cancer patients and in animal breast cancer models, obesity is a sufficient cause for increased expression of the bioactive sphingolipid mediator sphingosine-1-phosphate (S1P), which mediates cancer pathogenesis. A high-fat diet was sufficient to upregulate expression of sphingosine kinase 1 (SphK1), the enzyme that produces S1P, along with its receptor S1PR1 in syngeneic and spontaneous breast tumors. Targeting the SphK1/S1P/S1PR1 axis with FTY720/fingolimod attenuated key proinflammatory cytokines, macrophage infiltration, and tumor progression induced by obesity. S1P produced in the lung premetastatic niche by tumor-induced SphK1 increased macrophage recruitment into the lung and induced IL6 and signaling pathways important for lung metastatic colonization. Conversely, FTY720 suppressed IL6, macrophage infiltration, and S1P-mediated signaling pathways in the lung induced by a high-fat diet, and it dramatically reduced formation of metastatic foci. In tumor-bearing mice, FTY720 similarly reduced obesity-related inflammation, S1P signaling, and pulmonary metastasis, thereby prolonging survival. Taken together, our results establish a critical role for circulating S1P produced by tumors and the SphK1/S1P/S1PR1 axis in obesity-related inflammation, formation of lung metastatic niches, and breast cancer metastasis, with potential implications for prevention and treatment.Significance: These findings offer a preclinical proof of concept that signaling by a sphingolipid may be an effective target to prevent obesity-related breast cancer metastasis. Cancer Res; 78(7); 1713-25. ©2018 AACR.
©2018 American Association for Cancer Research.
Conflict of interest statement
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Figures




References
-
- Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA 2010;303: 235–41. - PubMed
-
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625–38. - PubMed
-
- Niraula S, Ocana A, Ennis M, Goodwin PJ. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: a meta-analysis. Breast Cancer Res Treat 2012;134:769–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

