EDC IMPACT: Molecular effects of developmental FM 550 exposure in Wistar rat placenta and fetal forebrain
- PMID: 29351906
- PMCID: PMC5817967
- DOI: 10.1530/EC-17-0373
EDC IMPACT: Molecular effects of developmental FM 550 exposure in Wistar rat placenta and fetal forebrain
Abstract
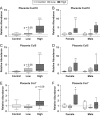
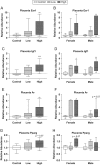
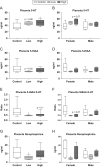
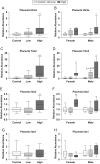
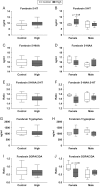
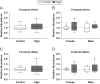
Firemaster 550 (FM 550) is a flame retardant (FR) mixture that has become one of the most commonly used FRs in foam-based furniture and baby products. Human exposure to this commercial mixture, composed of brominated and organophosphate components, is widespread. We have repeatedly shown that developmental exposure can lead to sex-specific behavioral effects in rats. Accruing evidence of endocrine disruption and potential neurotoxicity has raised concerns regarding the neurodevelopmental effects of FM 550 exposure, but the specific mechanisms of action remains unclear. Additionally, we observed significant, and in some cases sex-specific, accumulation of FM 550 in placental tissue following gestational exposure. Because the placenta is an important source of hormones and neurotransmitters for the developing brain, it may be a critical target of toxicity to consider in the context of developmental neurotoxicity. Using a mixture of targeted and exploratory approaches, the goal of the present study was to identify possible mechanisms of action in the developing forebrain and placenta. Wistar rat dams were orally exposed to FM 550 (0, 300 or 1000 µg/day) for 10 days during gestation and placenta and fetal forebrain tissue collected for analysis. In placenta, evidence of endocrine, inflammatory and neurotransmitter signaling pathway disruption was identified. Notably, 5-HT turnover was reduced in placental tissue and fetal forebrains indicating that 5-HT signaling between the placenta and the embryonic brain may be disrupted. These findings demonstrate that environmental contaminants, like FM 550, have the potential to impact the developing brain by disrupting normal placental functions.
Keywords: EDC; behavior; brain; endocrine disruptors; flame retardants; serotonin; sexually dimorphic.
© 2018 The authors.
Figures

References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

