Cost-effectiveness of Cognitive Behavioral Therapy for Depressed Youth Declining Antidepressants
- PMID: 29351965
- PMCID: PMC5810604
- DOI: 10.1542/peds.2017-1969
Cost-effectiveness of Cognitive Behavioral Therapy for Depressed Youth Declining Antidepressants
Abstract
Background and objectives: Adolescents with depression identified in primary care settings often have limited treatment options beyond antidepressant (AD) therapy. We assessed the cost-effectiveness of a brief cognitive behavioral therapy (CBT) program among depressed adolescents who declined or quickly stopped using ADs.
Methods: A total of 212 youth with depression were randomly assigned to treatment as usual (TAU) or TAU plus brief individual CBT. Clinical outcomes included depression-free days (DFDs) and estimated quality-adjusted life-years (QALYs). Costs were adjusted to 2008 US dollars. Incremental cost-effectiveness ratios (ICERs) comparing CBT to TAU were calculated over 12- and 24-month follow-up periods.
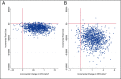
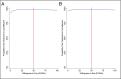
Results: Youth randomly assigned to CBT had 26.8 more DFDs (P = .044) and 0.067 more QALYs (P = .044) on average compared with TAU over 12 months. Total costs were $4976 less (P = .025) by the end of the 24-month follow-up among youth randomly assigned to CBT. Total costs per DFD were -$51 (ICER = -$51; 95% confidence interval [CI]: -$394 to $9) at 12 months and -$115 (ICER = -$115; 95% CI: -$1090 to -$6) at 24 months. Total costs per QALY were -$20 282 (ICER = -$20 282; 95% CI: -$156 741 to $3617) at 12 months and -$45 792 (ICER = -$45 792; 95% CI: -$440 991 to -$2731) at 24 months.
Conclusions: Brief primary care CBT among youth declining AD therapy is cost-effective by widely accepted standards in depression treatment. CBT becomes dominant over TAU over time, as revealed by a statistically significant cost offset at the end of the 2-year follow-up.
Trial registration: ClinicalTrials.gov NCT00523081.
Copyright © 2018 by the American Academy of Pediatrics.
Conflict of interest statement
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
Figures
References
-
- Gledhill J, Kramer T, Iliffe S, Garralda ME. Training general practitioners in the identification and management of adolescent depression within the consultation: a feasibility study. J Adolesc. 2003;26(2):245–250 - PubMed
-
- Rushton J, Bruckman D, Kelleher K. Primary care referral of children with psychosocial problems. Arch Pediatr Adolesc Med. 2002;156(6):592–598 - PubMed
-
- DeBar LL, Clarke GN, O’Connor E, Nichols GA. Treated prevalence, incidence, and pharmacotherapy of child and adolescent mood disorders in an HMO. Ment Health Serv Res. 2001;3(2):73–89 - PubMed
-
- Delate T, Gelenberg AJ, Simmons VA, Motheral BR. Trends in the use of antidepressants in a national sample of commercially insured pediatric patients, 1998 to 2002. Psychiatr Serv. 2004;55(4):387–391 - PubMed
-
- Clarke G, Dickerson J, Gullion CM, DeBar LL. Trends in youth antidepressant dispensing and refill limits, 2000 through 2009. J Child Adolesc Psychopharmacol. 2012;22(1):11–20 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

