Saa3 is a key mediator of the protumorigenic properties of cancer-associated fibroblasts in pancreatic tumors
- PMID: 29351990
- PMCID: PMC5819438
- DOI: 10.1073/pnas.1717802115
Saa3 is a key mediator of the protumorigenic properties of cancer-associated fibroblasts in pancreatic tumors
Abstract
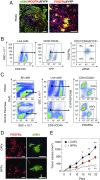
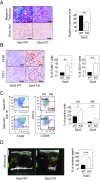
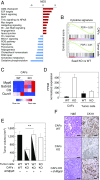
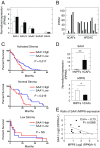
Pancreatic ductal adenocarcinoma (PDAC) is characterized by the presence of abundant desmoplastic stroma primarily composed of cancer-associated fibroblasts (CAFs). It is generally accepted that CAFs stimulate tumor progression and might be implicated in drug resistance and immunosuppression. Here, we have compared the transcriptional profile of PDGFRα+ CAFs isolated from genetically engineered mouse PDAC tumors with that of normal pancreatic fibroblasts to identify genes potentially implicated in their protumorigenic properties. We report that the most differentially expressed gene, Saa3, a member of the serum amyloid A (SAA) apolipoprotein family, is a key mediator of the protumorigenic activity of PDGFRα+ CAFs. Whereas Saa3-competent CAFs stimulate the growth of tumor cells in an orthotopic model, Saa3-null CAFs inhibit tumor growth. Saa3 also plays a role in the cross talk between CAFs and tumor cells. Ablation of Saa3 in pancreatic tumor cells makes them insensitive to the inhibitory effect of Saa3-null CAFs. As a consequence, germline ablation of Saa3 does not prevent PDAC development in mice. The protumorigenic activity of Saa3 in CAFs is mediated by Mpp6, a member of the palmitoylated membrane protein subfamily of the peripheral membrane-associated guanylate kinases (MAGUK). Finally, we interrogated whether these observations could be translated to a human scenario. Indeed, SAA1, the ortholog of murine Saa3, is overexpressed in human CAFs. Moreover, high levels of SAA1 in the stromal component correlate with worse survival. These findings support the concept that selective inhibition of SAA1 in CAFs may provide potential therapeutic benefit to PDAC patients.
Keywords: CAFs; PDAC; Saa3; mouse models; stroma.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. - PubMed
-
- Rahib L, et al. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. - PubMed
-
- Hidalgo M. Pancreatic cancer. N Engl J Med. 2010;362:1605–1617. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

