Direct cell-cell contact between mature osteoblasts and osteoclasts dynamically controls their functions in vivo
- PMID: 29352112
- PMCID: PMC5775424
- DOI: 10.1038/s41467-017-02541-w
Direct cell-cell contact between mature osteoblasts and osteoclasts dynamically controls their functions in vivo
Abstract
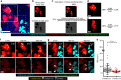
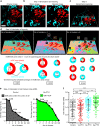
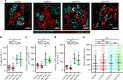
Bone homeostasis is regulated by communication between bone-forming mature osteoblasts (mOBs) and bone-resorptive mature osteoclasts (mOCs). However, the spatial-temporal relationship and mode of interaction in vivo remain elusive. Here we show, by using an intravital imaging technique, that mOB and mOC functions are regulated via direct cell-cell contact between these cell types. The mOBs and mOCs mainly occupy discrete territories in the steady state, although direct cell-cell contact is detected in spatiotemporally limited areas. In addition, a pH-sensing fluorescence probe reveals that mOCs secrete protons for bone resorption when they are not in contact with mOBs, whereas mOCs contacting mOBs are non-resorptive, suggesting that mOBs can inhibit bone resorption by direct contact. Intermittent administration of parathyroid hormone causes bone anabolic effects, which lead to a mixed distribution of mOBs and mOCs, and increase cell-cell contact. This study reveals spatiotemporal intercellular interactions between mOBs and mOCs affecting bone homeostasis in vivo.
Conflict of interest statement
T.I. and Y. Isogai are full-time employees of Asahi Kasei Pharma Corporation. The remaining authors declare no competing financial interests.
Figures



References
-
- Takahashi H, Epker BN, Frost HM. Resorption precedes formative activity. Surg. Forum. 1964;15:437–438. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

