PI3K/mTOR inhibition promotes the regression of experimental vascular malformations driven by PIK3CA-activating mutations
- PMID: 29352118
- PMCID: PMC5833448
- DOI: 10.1038/s41419-017-0064-x
PI3K/mTOR inhibition promotes the regression of experimental vascular malformations driven by PIK3CA-activating mutations
Abstract
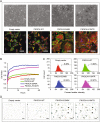
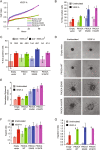
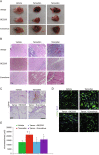
Somatic activating mutations within the PIK3CA gene have been recently detected in sporadic lymphatic and venous malformations, and in vascular malformations (VM) associated to overgrowth syndromes, such as CLOVES and Klippel-Trenaunay syndrome. Although VM are often limited to specific tissue areas and can be well treated, in extended or recurrent lesions novel therapeutic approaches are needed. We generated a mouse model of VM by local expression of PIK3CA-activating mutation in endothelial cells. PIK3CA-driven lesions are characterized by large areas of hemorrhage, hyperplastic vessels, infiltrates of inflammatory cells, and elevated endothelial cell density. Such vascular lesions are ameliorated by administration of dual PI3K/mTOR inhibitor, BEZ235, and mTOR inhibitor, Everolimus. Unexpectedly, the expression of PIK3CA-activating mutations in human endothelial cells results in both increased proliferation rates and senescence. Moreover, active forms of PIK3CA strongly promote the angiogenic sprouting. Treatment with PI3K/mTOR inhibitors restores normal endothelial cell proliferation rate and reduces the amount of senescent cells, whereas treatment with Akt inhibitor is less effective. Our findings reveal that PIK3CA mutations have a key role in the pathogenesis of VM and PIK3CA-driven experimental lesions can be effectively treated by PI3K/mTOR inhibitors.
Conflict of interest statement
Ethics approval and consent to participate
All experiments with animals were conducted according to the local regulations and with permission of local animal welfare officers.
Competing interests
The authors declare that they have no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

