Efficient differentiation of human pluripotent stem cells into skeletal muscle cells by combining RNA-based MYOD1-expression and POU5F1-silencing
- PMID: 29352121
- PMCID: PMC5775307
- DOI: 10.1038/s41598-017-19114-y
Efficient differentiation of human pluripotent stem cells into skeletal muscle cells by combining RNA-based MYOD1-expression and POU5F1-silencing
Abstract
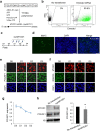
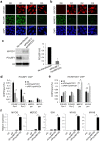
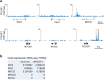
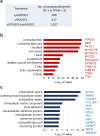
Direct generation of skeletal muscle cells from human pluripotent stem cells (hPSCs) would be beneficial for drug testing, drug discovery, and disease modelling in vitro. Here we show a rapid and robust method to induce myogenic differentiation of hPSCs by introducing mRNA encoding MYOD1 together with siRNA-mediated knockdown of POU5F1 (also known as OCT4 or OCT3/4). This integration-free approach generates functional skeletal myotubes with sarcomere-like structure and a fusion capacity in several days. The POU5F1 silencing facilitates MYOD1 recruitment to the target promoters, which results in the significant activation of myogenic genes in hPSCs. Furthermore, deep sequencing transcriptome analyses demonstrated that POU5F1-knockdown upregulates the genes associated with IGF- and FGF-signaling and extracellular matrix that may also support myogenic differentiation. This rapid and direct differentiation method may have potential applications in regenerative medicine and disease therapeutics for muscle disorders such as muscular dystrophy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

