Mitochondrial glutamine metabolism via GOT2 supports pancreatic cancer growth through senescence inhibition
- PMID: 29352139
- PMCID: PMC5833441
- DOI: 10.1038/s41419-017-0089-1
Mitochondrial glutamine metabolism via GOT2 supports pancreatic cancer growth through senescence inhibition
Abstract
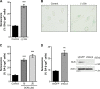
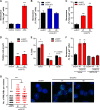
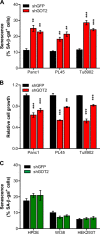
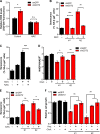
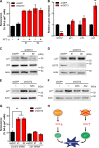
Cellular senescence, which leads to a cell cycle arrest of damaged or dysfunctional cells, is an important mechanism to restrain the malignant progression of cancer cells. Because metabolic changes underlie many cell-fate decisions, it has been suggested that cell metabolism might play key roles in senescence pathways. Here, we show that mitochondrial glutamine metabolism regulates senescence in human pancreatic ductal adenocarcinoma (PDAC) cells. Glutamine deprivation or inhibition of mitochondrial aspartate transaminase (GOT2) results in a profound induction of senescence and a suppression of PDAC growth. Glutamine carbon flow through GOT2 is required to create NADPH and to maintain the cellular redox state. We found that elevated reactive oxygen species levels by GOT2 knockdown lead to the cyclin-dependent kinase inhibitor p27-mediated senescence. Importantly, PDAC cells exhibit distinct dependence on this pathway, whereas knockdown of GOT2 did not induce senescence in non-transformed cells. The essentiality of GOT2 in senescence regulation of PDAC, which is dispensable in their normal counterparts, may have profound implications for the development of strategies to treat these refractory cancers.
Conflict of interest statement
The authors declare that they have no competing financial interests.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

