U-box ubiquitin ligase PPIL2 suppresses breast cancer invasion and metastasis by altering cell morphology and promoting SNAI1 ubiquitination and degradation
- PMID: 29352246
- PMCID: PMC5833831
- DOI: 10.1038/s41419-017-0094-4
U-box ubiquitin ligase PPIL2 suppresses breast cancer invasion and metastasis by altering cell morphology and promoting SNAI1 ubiquitination and degradation
Abstract
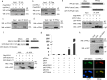
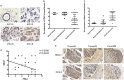
Metastasis is the leading cause of breast cancer fatalities. To develop new therapeutic strategies, the mechanisms underlying breast cancer invasion and metastasis need to be further investigated. Peptidylprolyl isomerase (cyclophilin)-like 2 (PPIL2) is a U-box-type E3 ubiquitin ligase belonging to the cyclophilin family. Proteins within this family are the major cytosolic binding proteins of the immunosuppressant drug cyclosporine A (CsA). Although PPIL2 has been reported to potentially be involved in cell migration, its role in breast cancer is still unclear. Herein, we demonstrate that PPIL2 suppressed metastasis in a breast cancer model by altering cell morphology and suppressing the epithelial-mesenchymal transition (EMT) process. Moreover, elevated PPIL2 inhibited EMT and breast cancer invasion by interacting with the classical EMT transcription factor, SNAI1, to enhance its ubiquitin-dependent degradation. Furthermore, PPIL2 protein level and stability was upregulated after CsA treatment, indicating that PPIL2 might be involved in CsA-mediated repression of EMT in breast cancer. Analysis of tissue samples taken from breast cancer patients showed a significant correlation between the expression of PPIL2 and the degree of cancer invasion and metastasis. In summary, these results would shed light on a potential clinical use of CsA in breast cancer patients.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

