Activation of PI3K, Akt, and ERK during early rotavirus infection leads to V-ATPase-dependent endosomal acidification required for uncoating
- PMID: 29352319
- PMCID: PMC5792019
- DOI: 10.1371/journal.ppat.1006820
Activation of PI3K, Akt, and ERK during early rotavirus infection leads to V-ATPase-dependent endosomal acidification required for uncoating
Abstract
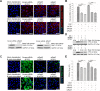
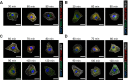
The cellular PI3K/Akt and/or MEK/ERK signaling pathways mediate the entry process or endosomal acidification during infection of many viruses. However, their roles in the early infection events of group A rotaviruses (RVAs) have remained elusive. Here, we show that late-penetration (L-P) human DS-1 and bovine NCDV RVA strains stimulate these signaling pathways very early in the infection. Inhibition of both signaling pathways significantly reduced production of viral progeny due to blockage of virus particles in the late endosome, indicating that neither of the two signaling pathways is involved in virus trafficking. However, immunoprecipitation assays using antibodies specific for pPI3K, pAkt, pERK and the subunit E of the V-ATPase co-immunoprecipitated the V-ATPase in complex with pPI3K, pAkt, and pERK. Moreover, Duolink proximity ligation assay revealed direct association of the subunit E of the V-ATPase with the molecules pPI3K, pAkt, and pERK, indicating that both signaling pathways are involved in V-ATPase-dependent endosomal acidification. Acidic replenishment of the medium restored uncoating of the RVA strains in cells pretreated with inhibitors specific for both signaling pathways, confirming the above results. Isolated components of the outer capsid proteins, expressed as VP4-VP8* and VP4-VP5* domains, and VP7, activated the PI3K/Akt and MEK/ERK pathways. Furthermore, psoralen-UV-inactivated RVA and CsCl-purified RVA triple-layered particles triggered activation of the PI3K/Akt and MEK/ERK pathways, confirming the above results. Our data demonstrate that multistep binding of outer capsid proteins of L-P RVA strains with cell surface receptors phosphorylates PI3K, Akt, and ERK, which in turn directly interact with the subunit E of the V-ATPase to acidify the late endosome for uncoating of RVAs. This study provides a better understanding of the RVA-host interaction during viral uncoating, which is of importance for the development of strategies aiming at controlling or preventing RVA infections.
Conflict of interest statement
The authors have declared that no competing interests exist
Figures









References
-
- Estes MK, Greenberg HB. Rotaviruses In: Fields Virology, 6th ed., eds Knipe DM, Howley PM et al. (Lippincott Williams & Wilkins, Philadelphia: ); 2013. pp. 1917–1974.
-
- Desselberger U. Rotaviruses. Virus Res. 2014; 190:75–96. doi: 10.1016/j.virusres.2014.06.016 . - DOI - PubMed
-
- Walker CL, Rudan I, Liu L, Nair H, Theodoratou E, Bhutta ZA, et al. Global burden of childhood pneumonia and diarrhea. Lancet. 2013; 381(9875):1405–1416. doi: 10.1016/S0140-6736(13)60222-6 . - DOI - PMC - PubMed
-
- Arias CF, Silva-Ayala D, López S. Rotavirus entry: a deep journey into the cell with several exits. J Virol. 2015; 89(2):890–893. doi: 10.1128/JVI.01787-14 . - DOI - PMC - PubMed
-
- López S, Arias CF. Multistep entry of rotavirus into cells: a Versaillesque dance. Trends Microbiol. 2004; 12(6):271–278. doi: 10.1016/j.tim.2004.04.003 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

