Crystal structure of ADP-dependent glucokinase from Methanocaldococcus jannaschii in complex with 5-iodotubercidin reveals phosphoryl transfer mechanism
- PMID: 29352744
- PMCID: PMC5818746
- DOI: 10.1002/pro.3377
Crystal structure of ADP-dependent glucokinase from Methanocaldococcus jannaschii in complex with 5-iodotubercidin reveals phosphoryl transfer mechanism
Abstract
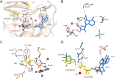
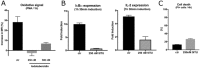
ADP-dependent glucokinase (ADPGK) is an alternative novel glucose phosphorylating enzyme in a modified glycolysis pathway of hyperthermophilic Archaea. In contrast to classical ATP-dependent hexokinases, ADPGK utilizes ADP as a phosphoryl group donor. Here, we present a crystal structure of archaeal ADPGK from Methanocaldococcus jannaschii in complex with an inhibitor, 5-iodotubercidin, d-glucose, inorganic phosphate, and a magnesium ion. Detailed analysis of the architecture of the active site allowed for confirmation of the previously proposed phosphorylation mechanism and the crucial role of the invariant arginine residue (Arg197). The crystal structure shows how the phosphate ion, while mimicking a β-phosphate group, is positioned in the proximity of the glucose moiety by arginine and the magnesium ion, thus providing novel insights into the mechanism of catalysis. In addition, we demonstrate that 5-iodotubercidin inhibits human ADPGK-dependent T cell activation-induced reactive oxygen species (ROS) release and downstream gene expression, and as such it may serve as a model compound for further screening for hADPGK-specific inhibitors.
Keywords: 5-iodotubercidin; ADP-dependent glucokinase; glycolysis; kinase inhibitor.
© 2018 The Protein Society.
Figures

References
-
- Kengen SW, Tuininga JE, de Bok FA, Stams AJ, de Vos WM (1995) Purification and characterization of a novel ADP‐dependent glucokinase from the hyperthermophilic archaeon Pyrococcus furiosus . J Biol Chem 270:30453–30457. - PubMed
-
- Ito S, Fushinobu S, Yoshioka I, Koga S, Matsuzawa H, Wakagi T (2001) Structural basis for the ADP‐specificity of a novel glucokinase from a hyperthermophilic archaeon. Structure 9:205–214. - PubMed
-
- Ito S, Fushinobu S, Jeong JJ, Yoshioka I, Koga S, Shoun H, Wakagi T (2003) Crystal structure of an ADP‐dependent glucokinase from Pyrococcus furiosus: implications for a sugar‐induced conformational change in ADP‐dependent kinase. J Mol Biol 331:871–883. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources

