Ectopic expression of the apple nucleus-encoded thylakoid protein MdY3IP1 triggers early-flowering and enhanced salt-tolerance in Arabidopsis thaliana
- PMID: 29352810
- PMCID: PMC5775602
- DOI: 10.1186/s12870-018-1232-6
Ectopic expression of the apple nucleus-encoded thylakoid protein MdY3IP1 triggers early-flowering and enhanced salt-tolerance in Arabidopsis thaliana
Abstract
Background: The roles in photosystem I (PSI) assembly of the nucleus-encoded thylakoid protein Y3IP1 who interacts with the plastid-encoded Ycf3 protein that has been well-characterized in plants. However, its function and potential mechanisms in other aspects remain poorly understood.
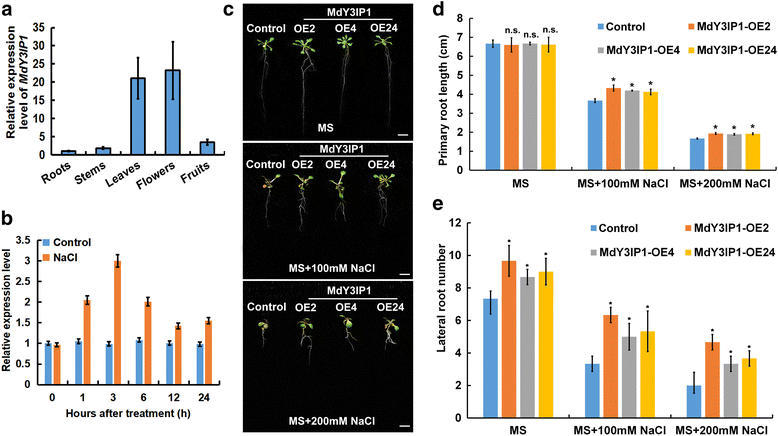
Results: We identified the apple MdY3IP1 gene, which encodes a protein highly homologous to the Arabidopsis Y3IP1 (AtY3IP1). Ectopic expression of MdY3IP1 triggered early-flowering and enhanced salt tolerance in Arabidopsis plants. MdY3IP1 controlled floral transition by accelerating sugar metabolism process in plant cells, thereby influencing the expression of flowering-associated genes. The increase in salt stress tolerance in MdY3IP1-expressing plants correlated with reduced reactive oxygen species (ROS) accumulation, and an increase in lateral root development by regulating both auxin biosynthesis and transport, as followed by enhancement of salt tolerance in Arabidopsis. Overall, these findings provide new evidences for additional functions of Y3IP1-like proteins and their underlying mechanisms of which Y3IP1 confers early-flowering and salt tolerance phenotypes in plants.
Conclusions: These observations suggest that plant growth and stress resistance can be affected by the regulation of the MdY3IP1 gene. Further molecular and genetic approaches will accelerate our knowledge of MdY3IP1 functions in PSI complex formation and plants stress resistance, and inform strategies for creating transgenic crop varieties with early maturity and high-resistant to adverse environmental conditions.
Keywords: Floral transition; MdY3IP1; PSI complex; ROS; Salt tolerance; Sugar metabolism.
Conflict of interest statement
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






References
-
- Biswal B, Krupinska K, Biswal UC. Plastid development in leaves during growth and senescence. 2013.
-
- Buchanan BB, Gruissem W, Jones RL. Biochemistry and molecular biology of plants. New York: Wiley; 2015. p. 1367.
MeSH terms
Substances
Grants and funding
- 31601728/National Natural Science Foundation of China/International
- 31325024 and 31430074/National Natural Science Foundation of China/International
- ZR2016CQ13 and SDAIT-06-03/Natural Science Foundation of Shandong Province/International
- 564024/Young Scientists Funds of Shandong Agricultural University/International
- 24024/Youth Science and Technology Innovation Fund of Shandong Agricultural University/International
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

