Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial
- PMID: 29353025
- PMCID: PMC8711034
- DOI: 10.1016/j.jaad.2018.01.016
Efficacy and safety of fezakinumab (an IL-22 monoclonal antibody) in adults with moderate-to-severe atopic dermatitis inadequately controlled by conventional treatments: A randomized, double-blind, phase 2a trial
Abstract
Background: Interleukin 22 promotes epidermal hyperplasia and inhibits skin barrier function.
Objective: Evaluate interleukin 22 blockade in adults with moderate-to-severe atopic dermatitis (AD).
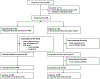
Methods: We performed a randomized, double-blind, placebo-controlled trial with intravenous fezakinumab monotherapy every 2 weeks for 10 weeks, with follow-up assessments until 20 weeks. The change in SCOring AD (SCORAD) score from baseline at 12 weeks served as the primary end point.
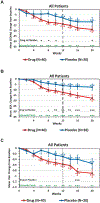
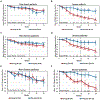
Results: At 12 weeks, the mean declines in SCORAD for the entire study population were 13.8 ± 2.7 in the fezakinumab arm and 8.0 ± 3.1 in the placebo arm (P = .134). In the severe AD patient subset (with a baseline SCORAD of ≥50), SCORAD decline was significantly stronger in the drug-treated patients than placebo-treated patients at 12 weeks (21.6 ± 3.8 vs 9.6 ± 4.2, P = .029) and 20 weeks (27.4 ± 3.9 vs 11.5 ± 5.1, P = .010). At 12 weeks, improvements in body surface area involvement in the entire population were significantly stronger in the drug-treated than placebo-treated patients (12.4% ± 2.4 vs 6.2% ± 2.7; P = .009), and in the severe AD subset, the decline in Investigator Global Assessment was significantly higher in the drug-treated than placebo-treated patients (0.7 ± 0.2 vs 0.3 ± 0.1; P = .034). All scores showed progressive improvements after last dosing (10 weeks) until end of study (20 weeks). Common adverse events were upper respiratory tract infections.
Limitations: The limited sample size and lack of assessment with Eczema Area and Severity Index and a pruritus numerical rating scale were limiting factors. Significance was primarily obtained in severe AD.
Conclusion: Fezakinumab was well-tolerated, with sustained clinical improvements after last drug dosing.
Keywords: IL-22; atopic dermatitis; fezakinumab; moderate-to-severe AD; placebo-controlled trial.
Copyright © 2018 American Academy of Dermatology, Inc. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
References
-
- Silverberg JI. Public Health Burden and Epidemiology of Atopic Dermatitis. Dermatol Clin 2017;35:283–9. - PubMed
-
- Weidinger S, Novak N. Atopic dermatitis. Lancet 2016;387:1109–22. - PubMed
-
- DaVeiga SP. Epidemiology of atopic dermatitis: a review. Allergy Asthma Proc 2012;33:227–34. - PubMed
-
- Drucker AM. Atopic dermatitis: Burden of illness, quality of life, and associated complications. Allergy Asthma Proc 2017;38:3–8. - PubMed
-
- Boguniewicz M, Alexis AF, Beck LA, et al. Expert Perspectives on Management of Moderate-to-Severe Atopic Dermatitis: A Multidisciplinary Consensus Addressing Current and Emerging Therapies. J Allergy Clin Immunol Pract 2017;5:1519–1531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

