Altered eicosanoid production and phospholipid remodeling during cell culture
- PMID: 29353239
- PMCID: PMC5832926
- DOI: 10.1194/jlr.M083030
Altered eicosanoid production and phospholipid remodeling during cell culture
Abstract
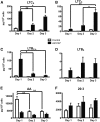
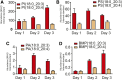
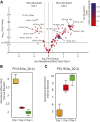
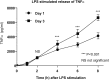
The remodeling of PUFAs by the Lands cycle is responsible for the diversity of phospholipid molecular species found in cells. There have not been detailed studies of the alteration of phospholipid molecular species as a result of serum starvation or depletion of PUFAs that typically occurs during tissue culture. The time-dependent effect of cell culture on phospholipid molecular species in RAW 264.7 cells cultured for 24, 48, or 72 h was examined by lipidomic strategies. These cells were then stimulated to produce arachidonate metabolites derived from the cyclooxygenase pathway, thromboxane B2, PGE2, and PGD2, and the 5-lipoxygenase pathway, leukotriene (LT)B4, LTC4, and 5-HETE, which decreased with increasing time in culture. However, the 5-lipoxygenase metabolites of a 20:3 fatty acid, LTB3, all trans-LTB3, LTC3, and 5-hydroxyeicosatrienoic acid, time-dependently increased. Molecular species of arachidonate containing phospholipids were drastically remodeled during cell culture, with a new 20:3 acyl group being populated into phospholipids to replace increasingly scarce arachidonate. In addition, the amount of TNFα induced by lipopolysaccharide stimulation was significantly increased in the cells cultured for 72 h compared with 24 h, suggesting that the remodeling of PUFAs enhanced inflammatory response. These studies supported the rapid operation of the Lands cycle to maintain cell growth and viability by populating PUFA species; however, without sufficient n-6 fatty acids, 20:3 n-9 accumulated, resulting in altered lipid mediator biosynthesis and inflammatory response.
Keywords: Mead acid; polyunsaturated fatty acid; volcano plot.
Copyright © 2018 by the American Society for Biochemistry and Molecular Biology, Inc.
Figures

References
-
- Burr G. O., and Burr M. M.. 1929. A new deficiency disease produced by the rigid exclusion of fat from the diet. J. Biol. Chem. 82: 345–367. - PubMed
-
- Farrell P. M., Gutcher G. R., Palta M., and DeMets D.. 1988. Essential fatty acid deficiency in premature infants. Am. J. Clin. Nutr. 48: 220–229. - PubMed
-
- Holman R. T. 1960. The ratio of trienoic: tetraenoic acids in tissue lipids as a measure of essential fatty acid requirement. J. Nutr. 70: 405–410. - PubMed
-
- Fulco A. J., and Mead J. F.. 1959. Metabolism of essential fatty acids. VIII. Origin of 5,8,11-eicosatrienoic acid in the fat-deficient rat. J. Biol. Chem. 234: 1411–1416. - PubMed
-
- Yamashita A., Hayashi Y., Nemoto-Sasaki Y., Ito M., Oka S., Tanikawa T., Waku K., and Sugiura T.. 2014. Acyltransferases and transacylases that determine the fatty acid composition of glycerolipids and the metabolism of bioactive lipid mediators in mammalian cells and model organisms. Prog. Lipid Res. 53: 18–81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

