The multiple roles of titin in muscle contraction and force production
- PMID: 29353351
- PMCID: PMC6082311
- DOI: 10.1007/s12551-017-0395-y
The multiple roles of titin in muscle contraction and force production
Abstract
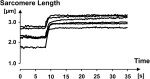
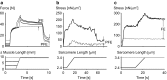
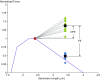
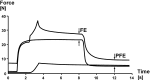
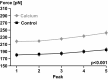
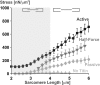
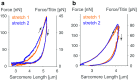
Titin is a filamentous protein spanning the half-sarcomere, with spring-like properties in the I-band region. Various structural, signaling, and mechanical functions have been associated with titin, but not all of these are fully elucidated and accepted in the scientific community. Here, I discuss the primary mechanical functions of titin, including its accepted role in passive force production, stabilization of half-sarcomeres and sarcomeres, and its controversial contribution to residual force enhancement, passive force enhancement, energetics, and work production in shortening muscle. Finally, I provide evidence that titin is a molecular spring whose stiffness changes with muscle activation and actin-myosin-based force production, suggesting a novel model of force production that, aside from actin and myosin, includes titin as a "third contractile" filament. Using this three-filament model of sarcomeres, the stability of (half-) sarcomeres, passive force enhancement, residual force enhancement, and the decrease in metabolic energy during and following eccentric contractions can be explained readily.
Keywords: Active/passive force regulation; Cross-bridge theory; Force production; Mechanical functions; Mechanisms of muscle contraction; Molecular spring; Muscle energetics; Muscle shortening; Three filament sarcomere model; Titin.
Conflict of interest statement
Walter Herzog declares that he has no conflict of interest.
Ethics approvals for all experiments described in this study were obtained by the Life Sciences and Animal Research Ethics Commitee of the University of Calgary.
Figures


References
-
- Allinger TL, Epstein M, Herzog W. Stability of muscle fibers on the descending limb of the force- length relation. A theoretical consideration. J Biomech. 1996;29:627–633. - PubMed
-
- Astier C, Raynaud F, Lebart MC, Roustan C, Benyamin Y. Binding of a native titin fragment to actin is regulated by PIP2. FEBS Lett. 1998;429:95–98. - PubMed
-
- Bartoo ML, Linke WA, Pollack GH. Basis of passive tension and stiffness in isolated rabbit myofibrils. Am J Phys. 1997;273:C266–C276. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

