Safety of axitinib and sorafenib monotherapy for patients with renal cell carcinoma: a meta-analysis
- PMID: 29353818
- PMCID: PMC5956256
- DOI: 10.7555/JBR.32.20170080
Safety of axitinib and sorafenib monotherapy for patients with renal cell carcinoma: a meta-analysis
Abstract
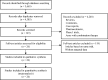
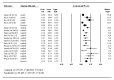
We sought to investigate safety of axitinib or sorafenib in renal cell carcinoma (RCC) patients and compare toxicity of these two vascular endothelial growth factor receptor inhibitors. Databases of PubMed and Embase were searched. We included phase II and III prospective trials, as well as retrospective studies, in which patients diagnosed with RCC were treated with axitinib or sorafenib monotherapy at a starting dose of 5 mg and 400 mg twice daily, respectively. The overall incidence of high grade hypertension, fatigue, gastrointestinal toxicity and hand-foot syndrome, along with their 95% confidence intervals (CI), were calculated using fixed- or random- effects model according to heterogeneity test results. A total of 26 trials, including 4790 patients, were included in our meta-analysis. Among them, 6 arms were related to axitinib and 22 were associated with sorafenib. The incidences of hypertension (24.9% vs. 7.9%), fatigue (8.2% vs. 6.6%), and gastrointestinal toxicity (17.6% vs. 11.3%) were higher in patients receiving axitinib versus those receiving sorafenib, while the incidence of hand-foot syndrome was lower in patients receiving axitinib versus those receiving sorafenib (9.5% vs. 13.3%). In conclusion, axitinib showed noticeably higher risks of toxicity versus sorafenib. Close monitoring and effective measures for adverse events are recommended during therapy.
Figures



References
-
- Rini BI, Campbell SC, Escudier B. Renal cell carcinoma[J]. Lancet, 2009, 373(9669): 1119–1132 . - PubMed
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics[J]. CA Cancer J Clin, 2016, 66(1): 7–30 . - PubMed
-
- Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial[J]. Lancet Oncol, 2013, 14(6): 552–562 . - PubMed
-
- Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial[J]. Lancet Oncol, 2013, 14(13): 1287–1294 . - PubMed
-
- Rini BI, Wilding G, Hudes G, et al. Phase II study of axitinib in sorafenib-refractory metastatic renal cell carcinoma[J]. J Clin Oncol, 2009, 27(27): 4462–4468 . - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

