Recent Progress in Decarboxylative Oxidative Cross-Coupling for Biaryl Synthesis
- PMID: 29354019
- PMCID: PMC5763354
- DOI: 10.1002/ejoc.201700121
Recent Progress in Decarboxylative Oxidative Cross-Coupling for Biaryl Synthesis
Abstract
The beginning of the 21st century has seen tremendous growth in the field of decarboxylative activation. Benzoic acid derivatives are now recognised as atom-economic alternatives to traditional cross-coupling partners, and they also benefit from being inexpensive, readily available and shelf-stable reagents. In this microreview we discuss recent developments in the coupling of benzoic acid derivatives either with an arene or with a second benzoic acid derivative, a process often termed decarboxylative oxidative cross-coupling. These procedures offer great promise for the development of highly selective and atom-economic cross-couplings.
Keywords: Biaryls; Cross‐coupling; C–H activation; Decarboxylation; Oxidative Coupling.
Figures



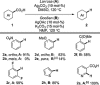










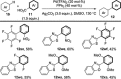











References
-
- For selected reviews on C–H activation, see: a) Alberico D., Scott M. E. and Lautens M., Chem. Rev., 2007, 107, 174–238; - PubMed
- b) Ackermann L., Vicente R. and Kapdi A. R., Angew. Chem. Int. Ed., 2009, 48, 9792–9826; - PubMed
- Angew. Chem., 2009, 121, 9976;
- c) Boorman T. C. and Larrosa I., Chem. Soc. Rev., 2011, 40, 1910–1925; - PubMed
- d) Wencel‐Delord J., Dröge T., Liu F. and Glorius F., Chem. Soc. Rev., 2011, 40, 4740–4761; - PubMed
- e) Kuhl N., Hopkinson M. N., Wencel‐Delord J. and Glorius F., Angew. Chem. Int. Ed., 2012, 51, 10236–10254; - PubMed
- Angew. Chem., 2012, 124, 10382;
- f) Kakiuchi F., Kochi T. and Murai S., Synlett, 2014, 25, 2390–2414;
- g) Ahlsten N., Cambeiro X. C., Perry G. J. P. and Larrosa I., Top. Heterocycl. Chem. 2016, 46, 175–226
-
- For selected reviews on decarboxylative activation, see: a) Gooßen L. J., Gooßen K., Rodríguez N., Blanchot M., Linder C. and Zimmermann B., Pure Appl. Chem., 2008, 80, 1725–1733;
- b) Gooßen L. J., Rodríguez N. and Gooßen K., Angew. Chem. Int. Ed., 2008, 47, 3100–3120; - PubMed
- Angew. Chem., 2008, 120, 3144;
- c) Gooßen L. J., Collet F. and Gooßen K., Isr. J. Chem., 2010, 50, 617–629;
- d) Shang R. and Liu L., Sci. China Chem., 2011, 54, 1670–1687;
- e) Rodríguez N. and Gooßen L. J., Chem. Soc. Rev., 2011, 40, 5030–5048; - PubMed
- f) Dzik W. I., Lange P. P. and Gooßen L. J., Chem. Sci., 2012, 3, 2671–2678;
- g) Cornella J. and Larrosa I., Synthesis, 2012, 44, 653–676;
- h) Gooßen L. J., Gooßen K., Reactions” “Decarboxylative Coupling, in: Topics in Organometallic Chemistry (Ed.: Gooßen L. J.), Springer‐Verlag, Berlin, Heidelberg, 2013, vol. 44, pp. 121–142.
-
- Shepard A. F., Winslow N. R. and Johnson J. R., J. Am. Chem. Soc., 1930, 52, 2083–2090.
-
- a) Nilsson M., Acta Chem. Scand., 1966, 20, 423–426;
- b) Chodowska‐Palicka J. and Nilsson M., Acta Chem. Scand., 1970, 24, 3353–3361.
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
