Beta and Gamma Human Herpesviruses: Agonistic and Antagonistic Interactions with the Host Immune System
- PMID: 29354096
- PMCID: PMC5760548
- DOI: 10.3389/fmicb.2017.02521
Beta and Gamma Human Herpesviruses: Agonistic and Antagonistic Interactions with the Host Immune System
Abstract
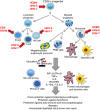
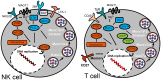
Viruses are the most abundant and diverse biological entities in the planet. Historically, our main interest in viruses has focused on their pathogenic role, recognized by pandemics that have decimated the world population. However, viral infections have also played a major role in the evolution of cellular organisms, both through interchanging of genes with novel functions and shaping the immune system. Examples abound of infections that seriously compromise the host integrity, but evidence of plant and insect viruses mutualistic relationships have recently surfaced in which infected hosts are better suited for survival, arguing that virus-host interactions are initially parasitic but become mutualistic over years of co-evolution. A similar mutual help scenario has emerged with commensal gut bacteria. EBV is a herpesvirus that shares more than a hundred million years of co-evolution with humans, today successfully infecting close to 100% of the adult world population. Infection is usually acquired early in childhood persisting for the host lifetime mostly without apparent clinical symptoms. Disturbance of this homeostasis is rare and results in several diseases, of which the best understood are infectious mononucleosis and several EBV-associated cancers. Less understood are recently found inborn errors of the immune system that result in primary immunodeficiencies with an increased predisposition almost exclusive to EBV-associated diseases. Puzzling to these scenarios of broken homeostasis is the co-existence of immunosuppression, inflammation, autoimmunity and cancer. Homologous to EBV, HCMV, HHV-6 and HHV-7 are herpesviruses that also latently infect most individuals. Several lines of evidence support a mutualistic equilibrium between HCMV/EBV and hosts, that when altered trigger diseases in which the immune system plays a critical role. Interestingly, these beta and gamma herpesviruses persistently infect all immune lineages and early precursor cells. In this review, we will discuss the evidence of the benefits that infection of immune cells with these herpesviruses brings to the host. Also, the circumstances in which this positive relationship is broken, predisposing the host to diseases characterized by an abnormal function of the host immune system.
Keywords: EBV; autoimmunity; herpesvirus; immunodeficiency; immunosuppression; inflammation; lymphoproliferation; mutualistic relationship.
Figures


References
-
- Agematsu K. (2000). Memory B cells and CD27. Histol. Histopathol. 15, 573–576. - PubMed
-
- Alkhairy O. K., Perez-Becker R., Driessen G. J., Abolhassani H., van Montfrans J., Borte S., et al. (2015). Novel mutations in TNFRSF7/CD27: clinical, immunologic, and genetic characterization of human CD27 deficiency. J. Allergy Clin. Immunol. 136, 703–12 e10. 10.1016/j.jaci.2015.02.022 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

