Insights into Structural and Mechanistic Features of Viral IRES Elements
- PMID: 29354113
- PMCID: PMC5759354
- DOI: 10.3389/fmicb.2017.02629
Insights into Structural and Mechanistic Features of Viral IRES Elements
Abstract
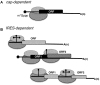
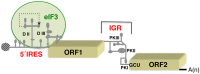
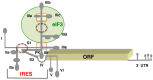
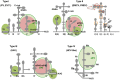
Internal ribosome entry site (IRES) elements are cis-acting RNA regions that promote internal initiation of protein synthesis using cap-independent mechanisms. However, distinct types of IRES elements present in the genome of various RNA viruses perform the same function despite lacking conservation of sequence and secondary RNA structure. Likewise, IRES elements differ in host factor requirement to recruit the ribosomal subunits. In spite of this diversity, evolutionarily conserved motifs in each family of RNA viruses preserve sequences impacting on RNA structure and RNA-protein interactions important for IRES activity. Indeed, IRES elements adopting remarkable different structural organizations contain RNA structural motifs that play an essential role in recruiting ribosomes, initiation factors and/or RNA-binding proteins using different mechanisms. Therefore, given that a universal IRES motif remains elusive, it is critical to understand how diverse structural motifs deliver functions relevant for IRES activity. This will be useful for understanding the molecular mechanisms beyond cap-independent translation, as well as the evolutionary history of these regulatory elements. Moreover, it could improve the accuracy to predict IRES-like motifs hidden in genome sequences. This review summarizes recent advances on the diversity and biological relevance of RNA structural motifs for viral IRES elements.
Keywords: IRES elements; RNA structure; RNA viruses; RNA-binding proteins; conserved RNA motifs.
Figures


References
-
- Andreev D. E., Fernandez-Miragall O., Ramajo J., Dmitriev S. E., Terenin I. M., Martinez-Salas E., et al. (2007). Differential factor requirement to assemble translation initiation complexes at the alternative start codons of foot-and-mouth disease virus RNA. RNA 13 1366–1374. 10.1261/rna.469707 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

