The Antioxidant Procyanidin Reduces Reactive Oxygen Species Signaling in Macrophages and Ameliorates Experimental Colitis in Mice
- PMID: 29354126
- PMCID: PMC5760499
- DOI: 10.3389/fimmu.2017.01910
The Antioxidant Procyanidin Reduces Reactive Oxygen Species Signaling in Macrophages and Ameliorates Experimental Colitis in Mice
Abstract
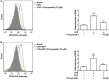
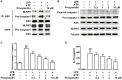
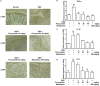
Management of inflammatory bowel disease (IBD) is a real clinical challenge. Despite intense investigation, the mechanisms of IBD remain substantially unidentified. Some inflammatory conditions, such as matrix metalloproteinases (MMPs) and the nuclear factor-κB (NF-κB) and NOD-like receptor protein 3 (NLRP3) inflammasome signaling pathways, are reported to contribute to the development and maintenance of IBD. Regulation of their common upstream signaling, that is, reactive oxygen species (ROS), may be important to control the progression of IBD. In the present study, we found that procyanidin, a powerful antioxidation flavonoid, has a significant effect on ROS clearance on THP-1 macrophages after lipopolysaccharide (LPS) or LPS-combined adenosine triphosphate stimulation, thus downregulating MMP9 expression, suppressing NF-κB signaling, and interrupting the formation of the NLRP3 inflammasome. Moreover, our in vivo data showed that procyanidin attenuated Dextran sulfate sodium-induced experimental colitis in a dose-dependent fashion by suppressing the expression of MMP9, NF-κB, and NLRP3 inflammasome signaling in colonic tissues in mice. Overall, our results suggested that targeting ROS could be a potential therapeutic choice for colonic inflammation.
Keywords: MMP9; NF-κB; NLRP3 inflammasome; inflammatory bowel disease; procyanidin; reactive oxygen species signaling.
Figures






References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

