Early Microglia Activation Precedes Photoreceptor Degeneration in a Mouse Model of CNGB1-Linked Retinitis Pigmentosa
- PMID: 29354133
- PMCID: PMC5760536
- DOI: 10.3389/fimmu.2017.01930
Early Microglia Activation Precedes Photoreceptor Degeneration in a Mouse Model of CNGB1-Linked Retinitis Pigmentosa
Abstract
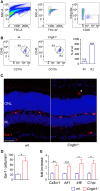
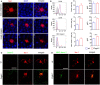
Retinitis pigmentosa (RP) denotes a family of inherited blinding eye diseases characterized by progressive degeneration of rod and cone photoreceptors in the retina. In most cases, a rod-specific genetic defect results in early functional loss and degeneration of rods, which is followed by degeneration of cones and loss of daylight vision at later stages. Microglial cells, the immune cells of the central nervous system, are activated in retinas of RP patients and in several RP mouse models. However, it is still a matter of debate whether activated microglial cells may be responsible for the amplification of the typical degenerative processes. Here, we used Cngb1-/- mice, which represent a slow degenerative mouse model of RP, to investigate the extent of microglia activation in retinal degeneration. With a combination of FACS analysis, immunohistochemistry and gene expression analysis we established that microglia in the Cngb1-/- retina were already activated in an early, predegenerative stage of the disease. The evidence available so far suggests that early retinal microglia activation represents a first step in RP, which might initiate or accelerate photoreceptor degeneration.
Keywords: cyclic nucleotide-gated channel; innate immune response; microglia; retinal degeneration; retinitis pigmentosa.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

