Impact of structurally modifying hyaluronic acid on CD44 interaction
- PMID: 29354263
- PMCID: PMC5773055
- DOI: 10.1039/C7TB01895A
Impact of structurally modifying hyaluronic acid on CD44 interaction
Abstract
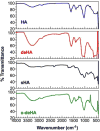
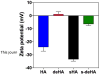
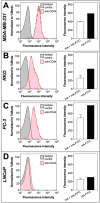
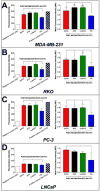
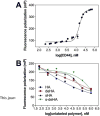
CD44 is a widely-distributed type I transmembrane glycoprotein that binds hyaluronic acid (HA) in most cell types, including primary tumor cells and cancer-initiating cells and has roles in cell migration, cell-cell, and cell-matrix adhesion. HA-derived conjugates and nanoparticles that target the CD44 receptor on cells have been reported for targeted delivery of therapeutics and imaging agents. Altering crucial interactions of HA with CD44 active sites holds significant importance in modulating targeting ability of hyaluronic acid to other cancer types that do not express the CD44 receptor or minimizing the interaction with CD44+ cells that are not target cells. The approach adopted here was deacetylation of the N-acetyl group and selective sulfation on the C6-OH on the HA polymer, which form critical interactions with the CD44 active site. Major interactions identified by molecular modeling were confirmed to be hydrogen bonding of the C6-OH with Tyr109 and hydrophobic interaction of the N-acetyl group with Tyr46, 83 and Ile 92. Modified HA was synthesized and characterized and its interactions were assessed by in vitro and molecular modeling approaches. In vitro techniques included flow cytometry and fluorescence polarization, while in silico approaches included docking and binding calculations by a MM-PBSA approach. These studies indicated that while both deacetylation and sulfation of HA individually decrease CD44 interaction, both chemical modifications are required to minimize interaction with CD44+ cells. The results of this study represent the first step to effective retargeting of HA-derived NPs for imaging and drug delivery.
Figures



References
-
- Lapcík L, Jr, Lapcík L, De Smedt S, Demeester J, Chabrecek P. Hyaluronan: Preparation, Structure, Properties, and Applications. Chem Rev. 1998;98(8):2663–2684. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

