The production of UL16-binding protein 1 targeted pigs using CRISPR technology
- PMID: 29354381
- PMCID: PMC5766454
- DOI: 10.1007/s13205-018-1107-4
The production of UL16-binding protein 1 targeted pigs using CRISPR technology
Erratum in
-
Correction to: The production of UL16-binding protein 1 targeted pigs using CRISPR technology.3 Biotech. 2018 Jul;8(7):316. doi: 10.1007/s13205-018-1334-8. Epub 2018 Jul 13. 3 Biotech. 2018. PMID: 30023148 Free PMC article.
Abstract
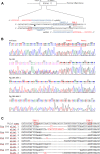
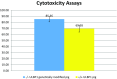
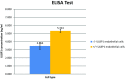
Two sgRNAs were designed to target the region of exon 2 of the pULBP1 gene by microinjection. The co-injection of modified Cas9-D10A nickase with a pair of sgRNAs into the zygote's cytoplasm easily and efficiently generated biallelic modification of the pULBP1 gene in one step. Five out of nine F0 generation piglets showed insertions or deletions in the targeting site of the pULBP1 gene, indicating that pULBP1 mutation efficiency reached about 56% (5/9). Quantitative determination of pULBP1 showed approximately a 1.53-fold reduction in the amount of protein ULBP1 on the cell surface (ELISA). A human NK-cell cytotoxicity test leads to the conclusion that higher cell viability is observed for -/- ULBP1 (survival rate 85.36%) compared to +/+ ULBP1 (69.58%). ULBP1-KO pigs will provide a more progressive xenograft source for further research studies, especially those measuring the effects of abolishing the gene function in terms of the complexity of the immunological interactions.
Keywords: CRISPR-Cas9; KO pigs; Microinjection; Xenotransplantation.
Conflict of interest statement
Compliance with ethical standardsAll authors declare that there is no conflict of interest.All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. Permission 1181/2015 from 21th May 2015 II Local Ethic Commission in Krakow).Not applicable.The data sets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Figures
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

