Polypharmacy in pediatric patients and opportunities for pharmacists' involvement
- PMID: 29354525
- PMCID: PMC5741016
- DOI: 10.2147/IPRP.S64535
Polypharmacy in pediatric patients and opportunities for pharmacists' involvement
Abstract
Rates of chronic conditions among pediatrics have been steadily increasing and medications used to treat these conditions have also shown a proportional increase. Most clinical trials focus on the safety of solitary medications in adult patients. However, data from these trials are often times extrapolated for use in pediatric patients who have different pharmacokinetic processes and physical profiles. As research increases and more drugs become available for pediatric use, the issue of polypharmacy becomes more of a concern. Polypharmacy is defined as the practice of administering or using multiple medications concurrently for the treatment of one to several medical disorders. With the increased rates of diagnosed complex disease states as prescribed mediations in pediatric patients, the prevalence and effect of polypharmacy in this patient population is largely a mystery. Polypharmacy falls within the realm of expertise of specialized pharmacists who can undertake medication therapy management services, medical chart reviews, and other services in pediatrics. Pharmacists have the time and knowledge to undertake pertinent interventions when managing polypharmacy and can play a major positive role in preventing adverse events. The aim of this paper is to review the literature on pediatric polypharmacy and provide insight into opportunities for pharmacists to help with management of polypharmacy. Information on adverse events, efficacy, and long-term outcomes with regard to growth and development of children subject to polypharmacy has yet to be published, leaving this realm of patient safety ripe for research.
Keywords: involvement; pediatrics; pharmacists; polypharmacy.
Conflict of interest statement
Disclosure The authors report no conflicts of interest in this work.
Figures
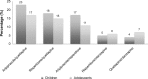
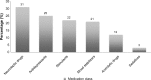
References
-
- Cox R, Halloran DR, Homan SM, Welliver S, Mager DE. Trends in the prevalence of chronic medication use in children: 2002–2005. Pediatrics. 2008;122:e1053–e1061. - PubMed
-
- Perrin JM, Anderson E, Van Cleave J. The rise in chronic conditions among infants, children, and youth can be met with continuous health system innovations. Health Affairs. 2014;33:2009–2105. - PubMed
-
- Wilson JT. An update on the therapeutic orphan. Pediatrics. 1999;104:585–590. - PubMed
-
- Roberts R, Rodriguez W, Murphy D, Crescenzi T. Pediatric drug labeling: improving the safety and efficacy of pediatric therapies. JAMA. 2003;290:905–911. - PubMed
-
- FDA.gov Food and Drug Administration Modernization Act of 1997. [Accessed March 8, 2015]. Available from: http://www.fda.gov/regulatoryinformation/legislation/federalfooddrugandc....
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

