Mutations in L-type amino acid transporter-2 support SLC7A8 as a novel gene involved in age-related hearing loss
- PMID: 29355479
- PMCID: PMC5811215
- DOI: 10.7554/eLife.31511
Mutations in L-type amino acid transporter-2 support SLC7A8 as a novel gene involved in age-related hearing loss
Abstract
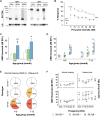
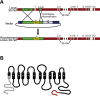
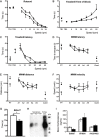
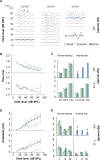
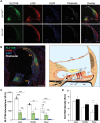
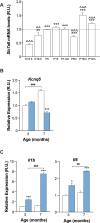
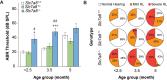
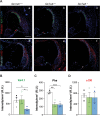
Age-related hearing loss (ARHL) is the most common sensory deficit in the elderly. The disease has a multifactorial etiology with both environmental and genetic factors involved being largely unknown. SLC7A8/SLC3A2 heterodimer is a neutral amino acid exchanger. Here, we demonstrated that SLC7A8 is expressed in the mouse inner ear and that its ablation resulted in ARHL, due to the damage of different cochlear structures. These findings make SLC7A8 transporter a strong candidate for ARHL in humans. Thus, a screening of a cohort of ARHL patients and controls was carried out revealing several variants in SLC7A8, whose role was further investigated by in vitro functional studies. Significant decreases in SLC7A8 transport activity was detected for patient's variants (p.Val302Ile, p.Arg418His, p.Thr402Met and p.Val460Glu) further supporting a causative role for SLC7A8 in ARHL. Moreover, our preliminary data suggest that a relevant proportion of ARHL cases could be explained by SLC7A8 mutations.
Keywords: LAT2; Slc7a8; age-related hearing loss; auditory brainstem response; chromosomes; genes; hearing loss; human; human biology; knock-out mouse model; medicine; mouse.
© 2018, Espino Guarch et al.
Conflict of interest statement
ME, MF, SM, EE, AC, GG, DV, MM, CV, SB, IS, LG, EP, AZ, MD, IV, PG, MP, VN No competing interests declared
Figures







Similar articles
-
Evidence for a relationship between genetic polymorphisms of the L-DOPA transporter LAT2/4F2hc and risk of hypertension in the context of chronic kidney disease.BMC Med Genomics. 2024 Jun 18;17(1):163. doi: 10.1186/s12920-024-01935-2. BMC Med Genomics. 2024. PMID: 38890684 Free PMC article.
-
Cooperation of Antiporter LAT2/CD98hc with Uniporter TAT1 for Renal Reabsorption of Neutral Amino Acids.J Am Soc Nephrol. 2018 Jun;29(6):1624-1635. doi: 10.1681/ASN.2017111205. Epub 2018 Apr 2. J Am Soc Nephrol. 2018. PMID: 29610403 Free PMC article.
-
New age-related hearing loss candidate genes in humans: an ongoing challenge.Gene. 2020 Jun 5;742:144561. doi: 10.1016/j.gene.2020.144561. Epub 2020 Mar 12. Gene. 2020. PMID: 32173538
-
Contributions of mouse models to understanding of age- and noise-related hearing loss.Brain Res. 2006 May 26;1091(1):89-102. doi: 10.1016/j.brainres.2006.03.017. Epub 2006 May 2. Brain Res. 2006. PMID: 16631134 Review.
-
Role of antioxidants in prevention of age-related hearing loss: a review of literature.Eur Arch Otorhinolaryngol. 2017 Apr;274(4):1821-1834. doi: 10.1007/s00405-016-4378-6. Epub 2016 Nov 17. Eur Arch Otorhinolaryngol. 2017. PMID: 27858145 Review.
Cited by
-
Choroid plexus LAT2 and SNAT3 as partners in CSF amino acid homeostasis maintenance.Fluids Barriers CNS. 2020 Feb 11;17(1):17. doi: 10.1186/s12987-020-0178-x. Fluids Barriers CNS. 2020. PMID: 32046769 Free PMC article.
-
LAT1-specific PET radiotracers: Development and clinical experiences of a new class of cancer-specific radiopharmaceuticals.Theranostics. 2025 Jan 2;15(5):1864-1878. doi: 10.7150/thno.99490. eCollection 2025. Theranostics. 2025. PMID: 39897549 Free PMC article. Review.
-
Structure of the human heterodimeric transporter 4F2hc-LAT2 in complex with Anticalin, an alternative binding protein for applications in single-particle cryo-EM.Sci Rep. 2022 Oct 30;12(1):18269. doi: 10.1038/s41598-022-23270-1. Sci Rep. 2022. PMID: 36310334 Free PMC article.
-
Insights on the role of thyroid hormone transport in neurosensory organs and implication for the Allan-Herndon-Dudley syndrome.Eur Thyroid J. 2024 Mar 19;13(2):e230241. doi: 10.1530/ETJ-23-0241. Print 2024 Apr 1. Eur Thyroid J. 2024. PMID: 38417253 Free PMC article. Review.
-
Dysfunctional LAT2 Amino Acid Transporter Is Associated With Cataract in Mouse and Humans.Front Physiol. 2019 Jun 4;10:688. doi: 10.3389/fphys.2019.00688. eCollection 2019. Front Physiol. 2019. PMID: 31231240 Free PMC article.
References
-
- Allen Institute for Brain Science 2004. Slc7a8 - RP_050915_02_F01 - sagittal. Allen Mouse Brain Atlas. 72001998
-
- Boettger T, Rust MB, Maier H, Seidenbecher T, Schweizer M, Keating DJ, Faulhaber J, Ehmke H, Pfeffer C, Scheel O, Lemcke B, Horst J, Leuwer R, Pape HC, Völkl H, Hübner CA, Jentsch TJ. Loss of K-Cl co-transporter KCC3 causes deafness, neurodegeneration and reduced seizure threshold. The EMBO Journal. 2003;22:5422–5434. doi: 10.1093/emboj/cdg519. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

