Involvement of cystatin C in immunity and apoptosis
- PMID: 29355583
- PMCID: PMC7112947
- DOI: 10.1016/j.imlet.2018.01.006
Involvement of cystatin C in immunity and apoptosis
Abstract
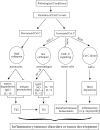
As an abundantly expressed cysteine protease inhibitor widely distributed in the organisms, cystatin C is involved in various physiological processes. Due to its relatively small molecular weight and easy detection, cystatin C is commonly used as a measure for glomerular filtration rate. In pathological conditions, however, growing evidences suggest that cystatin C is associated with various immune responses against either exogenous or endogenous antigens, which ultimately result in inflammatory autoimmune diseases or tumor development if not properly controlled. Thus the fluctuation of cystatin C levels might have more clinical implications than a reflection of kidney functions. Here, we summarize the latest development of studies on the pathophysiological functions of cystatin C, with focus on its immune regulatory roles at both cellular and molecular levels including antigen presentation, secretion of cytokines, synthesis of nitric oxide, as well as apoptosis. Finally, we discuss the clinical implications and therapeutic potentials of what this predominantly expressed protease inhibitor can bring to us.
Keywords: Apoptosis; Cystatin C; Cysteine proteases; Immune regulation; Inflammation.
Copyright © 2018 European Federation of Immunological Societies. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare no financial or commercial conflict of interest.
Figures

Similar articles
-
Functional characterization of Cystatin C in orange-spotted grouper, Epinephelus coioides.Dev Comp Immunol. 2019 Jul;96:37-46. doi: 10.1016/j.dci.2019.02.015. Epub 2019 Feb 27. Dev Comp Immunol. 2019. PMID: 30822452
-
Cystatin C is a disease-associated protein subject to multiple regulation.Immunol Cell Biol. 2015 May-Jun;93(5):442-51. doi: 10.1038/icb.2014.121. Epub 2015 Feb 3. Immunol Cell Biol. 2015. PMID: 25643616 Free PMC article. Review.
-
Molecular characterization, expression and functional analysis of cystatin C in Japanese flounder (Paralichthys olivaceus).Fish Shellfish Immunol. 2019 Mar;86:695-701. doi: 10.1016/j.fsi.2018.12.015. Epub 2018 Dec 10. Fish Shellfish Immunol. 2019. PMID: 30543934
-
Immunological Approaches Towards Cancer and Inflammation: A Cross Talk.Front Immunol. 2018 Mar 20;9:563. doi: 10.3389/fimmu.2018.00563. eCollection 2018. Front Immunol. 2018. PMID: 29662489 Free PMC article. Review.
-
Cystatin protease inhibitors and immune functions.Front Biosci. 2008 May 1;13:4625-37. doi: 10.2741/3028. Front Biosci. 2008. PMID: 18508534 Review.
Cited by
-
piggyBac Transposition and the Expression of Human Cystatin C in Transgenic Chickens.Animals (Basel). 2021 May 26;11(6):1554. doi: 10.3390/ani11061554. Animals (Basel). 2021. PMID: 34073441 Free PMC article.
-
Increased serum cystatin C levels and responses of pancreatic α- and β-cells in type 2 diabetes.Endocr Connect. 2022 Mar 21;11(3):e210597. doi: 10.1530/EC-21-0597. Endocr Connect. 2022. PMID: 35179515 Free PMC article.
-
Spatial localization of cathepsins: Implications in immune activation and resolution during infections.Front Immunol. 2022 Aug 3;13:955407. doi: 10.3389/fimmu.2022.955407. eCollection 2022. Front Immunol. 2022. PMID: 35990632 Free PMC article. Review.
-
Accelerated epigenetic aging and decreased natural killer cells based on DNA methylation in patients with untreated major depressive disorder.NPJ Aging. 2023 Sep 6;9(1):19. doi: 10.1038/s41514-023-00117-1. NPJ Aging. 2023. PMID: 37673891 Free PMC article.
-
Using different machine learning models to classify patients into mild and severe cases of COVID-19 based on multivariate blood testing.J Med Virol. 2022 Jan;94(1):357-365. doi: 10.1002/jmv.27352. Epub 2021 Sep 25. J Med Virol. 2022. PMID: 34542195 Free PMC article.
References
-
- Turk B., Turk D., Salvesen G.S. Regulating cysteine protease activity: essential role of protease inhibitors as guardians and regulators. Curr. Pharm. Des. 2002;8(18):1623–1637. - PubMed
-
- Keppler D. Towards novel anti-cancer strategies based on cystatin function. Cancer Lett. 2006;235(2):159–176. - PubMed
-
- Bobek L.A., Levine M.J. Cystatins–inhibitors of cysteine proteinases. Crit. Rev. Oral Biol. Med. 1992;3(4):307–332. - PubMed
-
- Georges S., Ruiz Velasco C., Trichet V., Fortun Y., Heymann D., Padrines M. Proteases and bone remodelling. Cytokine Growth Factor Rev. 2009;20(1):29–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

