Antithrombotic therapy in peripheral artery disease: A review of the EUCLID trial results and current ongoing trials
- PMID: 29355992
- PMCID: PMC6490002
- DOI: 10.1002/clc.22839
Antithrombotic therapy in peripheral artery disease: A review of the EUCLID trial results and current ongoing trials
Abstract
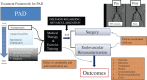
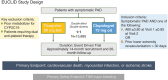
In addition to risk-factor modification, antithrombotic therapy is the hallmark of management to reduce cardiovascular ischemic events in patients with peripheral artery disease (PAD). Currently, the guidelines recommend long-term antiplatelet therapy with aspirin or clopidogrel in this patient population to reduce myocardial infarction, stroke, and vascular death. Past outcomes studies have shown some benefit of ticagrelor, another antiplatelet agent, as compared with clopidogrel in patients with coronary disease and concomitant PAD. However, most recently, the Examining Use of Ticagrelor in Peripheral Artery Disease (EUCLID) trial has shown no additional benefit of ticagrelor over clopidogrel. In this trial, a minority of patients had concomitant coronary artery disease, making it unique to previous studies. The EUCLID trial's evidence of neutrality between clopidogrel and ticagrelor sheds light into the complexity of studying the PAD population and the continued need to meticulously design trials to investigate the optimal therapies. The topics that will be discussed in this review include the role of antiplatelet therapy in the management of patients with PAD, a review of the EUCLID trial results and the important factors to be considered in interpreting the surprising results, and promising recent ongoing clinical trials assessing therapies in the treatment of patients with PAD.
Keywords: Antithrombotic Therapy; Aspirin; Clopidogrel; Peripheral Artery Disease; Peripheral Vascular Intervention.
© 2018 Wiley Periodicals, Inc.
Conflict of interest statement
Dr. Patel reports research grants from AstraZeneca and honorarium/other support from Bayer and Janssen Pharmaceuticals. Dr. Jones reports research grants from the Agency for Healthcare Research and Quality, AstraZeneca, American Heart Association, Bristol‐Myers Squibb, Doris Duke Charitable Foundation, and Patient‐Centered Outcomes Research Institute; and honorarium/other support from the American College of Radiology, Bayer, Bristol‐Myers Squibb, Daiichi Sankyo, and Janssen Pharmaceuticals. The authors declare no other potential conflicts of interest.
Figures
References
-
- Creager MA, Belkin M, Bluth EI, et al. 2012 ACCF/AHA/ACR/SCAI/SIR/STS/SVM/SVN/SVS Key data elements and definitions for peripheral atherosclerotic vascular disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Clinical Data Standards (Writing Committee to develop Clinical Data Standards for peripheral atherosclerotic vascular disease) [published corrections appear in J Am Coll Cardiol. 2012;59:702 and 2013;62:1042]. J Am Coll Cardiol. 2012;59:294–357. - PubMed
-
- Norgren L, Hiatt WR, Dormandy JA, et al. Inter‐Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J Vasc Surg. 2007;45(suppl):S5–S67. - PubMed
-
- 2011 Writing Group Members, 2005 Writing Committee Members, ACCF/AHA Task Force Members . ACCF/AHA Focused Update of the Guideline for the management of patients with peripheral artery disease (updating the 2005 Guideline): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2011;124:2020–2045. - PubMed
-
- Harris LM, Peer R, Curl GR, et al. Long‐term follow‐up of patients with early atherosclerosis. J Vasc Surg. 1996;23:576–581. - PubMed
-
- Sigvant B, Hasvold P, Kragsterman B, et al. Cardiovascular outcomes in patients with peripheral arterial disease as an initial or subsequent manifestation of atherosclerotic disease: results from a Swedish nationwide study. J Vasc Surg. 2017;66:507.e1–514.e1. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

