Familial Congenital Methemoglobinemia in Pomeranian Dogs Caused by a Missense Variant in the NADH-Cytochrome B5 Reductase Gene
- PMID: 29356095
- PMCID: PMC5787195
- DOI: 10.1111/jvim.15031
Familial Congenital Methemoglobinemia in Pomeranian Dogs Caused by a Missense Variant in the NADH-Cytochrome B5 Reductase Gene
Abstract
Background: In veterinary medicine, congenital methemoglobinemia associated with nicotinamide adenine dinucleotide (NADH)-cytochrome b5 reductase (b5R) deficiency is rare. It has been reported in several breeds of dogs, but little information is available about its etiology.
Objectives: To analyze the NADH-cytochrome b5 reductase gene, CYB5R3, in a Pomeranian dog family with methemoglobinemia suspected to be caused by congenital b5R deficiency.
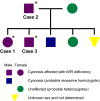
Animals: Three Pomeranian dogs from a family with methemoglobinemia were analyzed. Five healthy beagles and 5 nonrelated Pomeranian dogs without methemoglobinemia were used as controls.
Methods: Methemoglobin concentration, b5R activity, and reduced glutathione (GSH) concentration were measured, and a turbidity index was used to evaluate Heinz body formation. The CYB5R3 genes of the affected dog and healthy dogs were analyzed by direct sequencing.
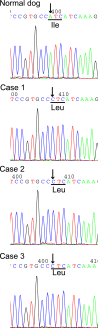
Results: Methemoglobin concentrations in erythrocytes of the affected dogs were remarkably higher than those of the control dogs. The b5R activity of the affected dogs was notably lower than that of the control dogs. DNA sequencing indicated that this Pomeranian family carried a CYB5R3 gene missense variant (ATC→CTC at codon 194) that resulted in the replacement of isoleucine (Ile) by leucine (Leu).
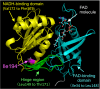
Conclusions and clinical importance: This dog family had familial congenital methemoglobinemia caused by b5R deficiency, which resulted from a nonsynonymous variant in the CYB5R3 gene. This variation (c.580A>C) led to an amino acid substitution (p.Ile194Leu), and Ile194 was located in the proximal region of the NADH-binding motif. Our data suggested that this variant in the canine CYB5R3 gene would affect function of the b5R in erythrocytes.
Keywords: CYB5R3 gene; Familial methemoglobinemia; Missense variant; NADH-cytochrome b5 reductase deficiency; Nonsynonymous SNP.
Copyright © 2018 The Authors. Journal of Veterinary Internal Medicine published by Wiley Periodicals, Inc. on behalf of the American College of Veterinary Internal Medicine.
Conflict of interest statement
The authors declare that they have no conflict of interests.
Figures

Similar articles
-
Characterization of a novel nicotinamide adenine dinucleotide-cytochrome b5 reductase mutation associated with canine hereditary methemoglobinemia.J Vet Med Sci. 2021 Mar 5;83(2):315-321. doi: 10.1292/jvms.20-0390. Epub 2020 Dec 21. J Vet Med Sci. 2021. PMID: 33342963 Free PMC article.
-
Genetic cause for congenital methemoglobinemia in an Australian Pomeranian dog.J Vet Intern Med. 2019 Mar;33(2):868-873. doi: 10.1111/jvim.15435. Epub 2019 Feb 14. J Vet Intern Med. 2019. PMID: 30767280 Free PMC article.
-
Long-term Treatment with Methylene Blue in a Dog with Hereditary Methemoglobinemia Caused by Cytochrome b5 Reductase Deficiency.J Vet Intern Med. 2017 Nov;31(6):1860-1865. doi: 10.1111/jvim.14843. Epub 2017 Sep 29. J Vet Intern Med. 2017. PMID: 28963729 Free PMC article.
-
Recessive congenital methaemoglobinaemia: cytochrome b(5) reductase deficiency.Br J Haematol. 2008 May;141(3):298-308. doi: 10.1111/j.1365-2141.2008.07017.x. Epub 2008 Mar 3. Br J Haematol. 2008. PMID: 18318771 Review.
-
Methemoglobin pathophysiology.Prog Clin Biol Res. 1981;51:133-51. Prog Clin Biol Res. 1981. PMID: 7022466 Review.
Cited by
-
Circulating Methemoblogin Fraction in Dogs With Sepsis.Front Vet Sci. 2020 Jun 16;7:341. doi: 10.3389/fvets.2020.00341. eCollection 2020. Front Vet Sci. 2020. PMID: 32656253 Free PMC article.
-
Hereditary methemoglobinemia in a cyanotic cat presented for ovariohysterectomy.Can Vet J. 2019 May;60(5):502-506. Can Vet J. 2019. PMID: 31080263 Free PMC article.
-
Dog-human translational genomics: state of the art and genomic resources.J Appl Genet. 2022 Dec;63(4):703-716. doi: 10.1007/s13353-022-00721-z. Epub 2022 Sep 8. J Appl Genet. 2022. PMID: 36074326 Review.
-
Clinical, metabolic, and molecular genetic characterization of hereditary methemoglobinemia caused by cytochrome b5 reductase deficiency in 30 dogs.Sci Rep. 2020 Dec 8;10(1):21399. doi: 10.1038/s41598-020-78391-2. Sci Rep. 2020. PMID: 33293645 Free PMC article.
-
Transient methemoglobinemia suspected secondary to ingestion of Brassica species in a dog.Vet Med (Auckl). 2019 Apr 29;10:37-42. doi: 10.2147/VMRR.S195458. eCollection 2019. Vet Med (Auckl). 2019. PMID: 31119092 Free PMC article.
References
-
- Key DM, Kelly JH. Methemoglobinemia in a dog. Vet Med Small Anim Clin 1980;75:245–246. - PubMed
-
- Wright RO, Lewander WJ, Woolf AD. Methemoglobinemia: Etiology, pharmacology, and clinical management. Ann Emerg Med 1999;34:646–656. - PubMed
-
- Rockwood GA, Armstrong KR, Baskin SI. Species comparison of methemoglobin reductase. Exp Biol Med 2003;228:79–83. - PubMed
-
- Harvey JW. Pathogenesis, laboratory diagnosis and clinical implications of erythrocyte enzyme deficiencies in dogs, cats and horses. Vet Clin Pathol 2006;35:144–156. - PubMed
-
- Percy MJ, Lappin TR. Recessive congenital methaemoglobinaemia: cytochrome b(5) reductase deficiency. Br J Haematol 2008;141:298–308. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

