Emerging Strategies of Cancer Therapy Based on Ferroptosis
- PMID: 29356212
- PMCID: PMC6377162
- DOI: 10.1002/adma.201704007
Emerging Strategies of Cancer Therapy Based on Ferroptosis
Abstract
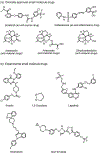
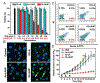
Ferroptosis, a new form of regulated cell death that is iron- and reactive oxygen species dependent, has attracted much attention in the research communities of biochemistry, oncology, and especially material sciences. Since the first demonstration in 2012, a series of strategies have been developed to induce ferroptosis of cancer cells, including the use of nanomaterials, clinical drugs, experimental compounds, and genes. A plethora of research work has outlined the blueprint of ferroptosis as a new option for cancer therapy. However, the published ferroptosis-related reviews have mainly focused on the mechanisms and pathways of ferroptosis, which motivated this contribution to bridge the gap between biological significance and material design. Therefore, it is timely to summarize the previous efforts on the emerging strategies for inducing ferroptosis and shed light on future directions for using such a tool to fight against cancer. Here, the current strategies of cancer therapy based on ferroptosis will be elaborated, the design considerations and the advantages and limitations are highlighted, and finally a future perspective on this emerging field is given.
Keywords: Fenton reaction; cancer therapy; ferroptosis; iron-based nanomaterials; lipid peroxidation.
© 2018 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures



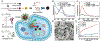






References
-
- Tan S, Schubert D, Maher P, Curr. Top. Med. Chem 2001, 1, 497. - PubMed
-
- Doll S, Conrad M, IUBMB Life 2017, 69, 423. - PubMed
-
- Seiler A, Schneider M, Forster H, Roth S, Wirth EK, Culmsee C, Plesnila N, Kremmer E, Radmark O, Wurst W, Cell Metab 2008, 8, 237. - PubMed
-
- Dolma S, Lessnick SL, Hahn WC, Stockwell BR, Cancer Cell 2003, 3, 285. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

