Site-Specifically Labeled Antibody-Drug Conjugate for Simultaneous Therapy and ImmunoPET
- PMID: 29356543
- PMCID: PMC6466629
- DOI: 10.1021/acs.molpharmaceut.7b00802
Site-Specifically Labeled Antibody-Drug Conjugate for Simultaneous Therapy and ImmunoPET
Abstract
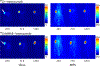
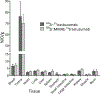
The conjugation of antibodies with cytotoxic drugs can alter their in vivo pharmacokinetics. As a result, the careful assessment of the in vivo behavior, and specifically the tumor-targeting properties, of antibody-drug conjugates represents a crucial step in their development. In order to facilitate this process, we have created a methodology that facilitates the dual labeling of an antibody with both a toxin and a radionuclide for positron emission tomography (PET). To minimize the impact of these modifications, this chemoenzymatic approach leverages strain-promoted azide-alkyne click chemistry to graft both cargoes to the heavy chain glycans of the immuoglobulin's Fc domain. As a proof-of-concept, a HER2-targeting trastuzumab immunoconjugate was created bearing both a monomethyl auristatin E (MMAE) toxin as well as the long-lived positron-emitting radiometal 89Zr ( t1/2 ≈ 3.3 days). Both the tumor targeting and therapeutic efficacy of the 89Zr-trastuzumab-MMAE immunoconjugate were validated in vivo using a murine model of HER2-expressing breast cancer. The site-specifically dual-labeled construct enabled the clear visualization of tumor tissue via PET imaging, producing tumoral uptake of ∼70%ID/g. Furthermore, a longitudinal therapy study revealed that the immunoconjugate exerts significant antitumor activity, leading to a >90% reduction in tumor volume over the course of 20 days.
Keywords: ADC; PET; antibody−drug conjugate; click chemistry; heavy chain glycans; positron emission tomography; site-specific bioconjugation; strain-promoted azide−alkyne click chemistry.
Figures
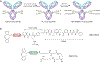


References
-
- https://www.ncbi.nlm.nih.gov/pubmed/?term=antibody-drug+conjugates (2017, January 19) antibody-drug conjugates - PubMed - NCBI.
-
- Phillips GDL, Li G, Dugger DL, Crocker LM, Parsons KL, Mai E, Blättler WA, Lambert JM, Chari RVJ, Lutz RJ, et al. (2008) Targeting HER2-Positive Breast Cancer with Trastuzumab-DM1, an Antibody–Cytotoxic Drug Conjugate. Cancer Res. 68, 9280–9290. - PubMed
-
- Merten H, Brandl F, Plückthun A, and Zangemeister-Wittke U (2015) Antibody–Drug Conjugates for Tumor Targeting—Novel Conjugation Chemistries and the Promise of non-IgG Binding Proteins. Bioconjug. Chem. 26, 2176–2185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

