3,5-Dicaffeoylquinic Acid Disperses Aspergillus Fumigatus Biofilm and Enhances Fungicidal Efficacy of Voriconazole and Amphotericin B
- PMID: 29356802
- PMCID: PMC5788051
- DOI: 10.12659/msm.908068
3,5-Dicaffeoylquinic Acid Disperses Aspergillus Fumigatus Biofilm and Enhances Fungicidal Efficacy of Voriconazole and Amphotericin B
Abstract
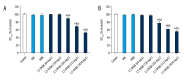
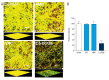
BACKGROUND The aim of this study was to evaluate the dispersal effects of 3,5-dicaffeoylquinic acid (3,5-DCQA) against the preformed biofilm of Aspergillus fumigatus and to investigate its potential mechanism. MATERIAL AND METHODS Aspergillus fumigatus biofilms of laboratory strain AF293 and clinical strain GXMU04 were generated in 24- or 96-well polystyrene microtiter plates in vitro. Crystal violet assay and XTT reduction assay were performed to evaluate the effects of 3,5-DCQA on biofilm biomass, extracellular matrix, and metabolic activity alteration of cells in biofilms. Real-time PCR was performed to quantify the expression of hydrophobin genes. The cytotoxicity of 3,5-DCQA on human erythrocytes was evaluated by a hemolytic assay. RESULTS The results indicated that 3,5-DCQA in subminimum inhibitory concentrations (256 to 1024 mg/L) elicited optimal A. fumigatus biofilm dispersion activity and improved the efficacy of VRC and AMB in minimal fungicidal concentrations (MFCs) to combat fungal cells embedded in biofilms. The results of scanning electron microscope (SEM) and confocal laser scanning microscopy (CLSM) revealed 3,5-DCQA facilitated the entry of antifungal agents into the A. fumigatus biofilm through eliminating the hydrophobic extracellular matrix (ECM) without affecting fungal growth. Real-time PCR indicated that 3,5-DCQA down-regulated the expression of hydrophobin genes. Hemolytic assay confirmed that 3,5-DCQA exhibited a low cytotoxicity against human erythrocytes. CONCLUSIONS Subminimum inhibitory concentrations of 3,5-DCQA can disperse A. fumigatus biofilm and enhance fungicidal efficacy of VRC and AMB through down-regulating expression of the hydrophobin genes. The study indicated the anti-biofilm potential of 3,5-DCQA for the management of A. fumigatus biofilm-associated infection.
Conflict of interest statement
None.
Figures




Similar articles
-
Prior in vitro exposure to voriconazole confers resistance to amphotericin B in Aspergillus fumigatus biofilms.Int J Antimicrob Agents. 2015 Sep;46(3):342-5. doi: 10.1016/j.ijantimicag.2015.03.006. Epub 2015 Apr 24. Int J Antimicrob Agents. 2015. PMID: 25979638
-
Aspergillus fumigatus DBM 4057 biofilm formation is inhibited by chitosan, in contrast to baicalein and rhamnolipid.World J Microbiol Biotechnol. 2016 Nov;32(11):187. doi: 10.1007/s11274-016-2146-9. Epub 2016 Sep 22. World J Microbiol Biotechnol. 2016. PMID: 27660214
-
In vitro interaction between alginate lyase and amphotericin B against Aspergillus fumigatus biofilm determined by different methods.Antimicrob Agents Chemother. 2013 Mar;57(3):1275-82. doi: 10.1128/AAC.01875-12. Epub 2012 Dec 21. Antimicrob Agents Chemother. 2013. PMID: 23263007 Free PMC article.
-
The characteristics of Aspergillus fumigatus mycetoma development: is this a biofilm?Med Mycol. 2009;47 Suppl 1:S120-6. doi: 10.1080/13693780802238834. Epub 2008 Jul 24. Med Mycol. 2009. PMID: 18654926 Review.
-
Aspergillus fumigatus biofilms in the clinical setting.Med Mycol. 2011 Apr;49 Suppl 1:S96-S100. doi: 10.3109/13693786.2010.502190. Epub 2011 Jan 24. Med Mycol. 2011. PMID: 21254964 Review.
Cited by
-
Azygos Vein Aneurysm with Thrombosis and Aspergillus fumigatus Diagnosed Using Bronchoscopy: Case Report.Am J Case Rep. 2020 Jul 29;21:e923401. doi: 10.12659/AJCR.923401. Am J Case Rep. 2020. PMID: 32726301 Free PMC article.
-
Microbial functional amyloids serve diverse purposes for structure, adhesion and defence.Biophys Rev. 2019 Jun;11(3):287-302. doi: 10.1007/s12551-019-00526-1. Epub 2019 May 2. Biophys Rev. 2019. PMID: 31049855 Free PMC article. Review.
-
Allicin shows antifungal efficacy against Cryptococcus neoformans by blocking the fungal cell membrane.Front Microbiol. 2022 Nov 16;13:1012516. doi: 10.3389/fmicb.2022.1012516. eCollection 2022. Front Microbiol. 2022. PMID: 36466672 Free PMC article.
-
Modulated Response of Aspergillus fumigatus and Stenotrophomonas maltophilia to Antimicrobial Agents in Polymicrobial Biofilm.Front Cell Infect Microbiol. 2020 Oct 6;10:574028. doi: 10.3389/fcimb.2020.574028. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33123497 Free PMC article.
-
Phytotoxic and Antifungal Effects of Plantago major and Sambucus nigra Bioextracts on Key Agricultural Pathogens: Corynespora cassiicola, Fusarium oxysporum, and Penicillium oxalicum.Pathogens. 2025 Feb 7;14(2):162. doi: 10.3390/pathogens14020162. Pathogens. 2025. PMID: 40005537 Free PMC article.
References
-
- Mowat E, Butcher J, Lang S, et al. Development of a simple model for studying the effects of antifungal agents on multicellular communities of Aspergillus fumigatus. J Med Microbiol. 2007;56(Pt 9):1205–12. - PubMed
-
- Muller FM, Seidler M, Beauvais A. Aspergillus fumigatus biofilms in the clinical setting. Med Mycol. 2011;49(Suppl 1):S96–S100. - PubMed
-
- Kaur S, Singh S. Biofilm formation by Aspergillus fumigatus. Med Mycol. 2014;52(1):2–9. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

