Association of Time to Treatment With Short-term Outcomes for Pediatric Patients With Refractory Convulsive Status Epilepticus
- PMID: 29356811
- PMCID: PMC5885266
- DOI: 10.1001/jamaneurol.2017.4382
Association of Time to Treatment With Short-term Outcomes for Pediatric Patients With Refractory Convulsive Status Epilepticus
Abstract
Importance: Treatment delay for seizures can lead to longer seizure duration. Whether treatment delay is associated with major adverse outcomes, such as death, remains unknown.
Objective: To evaluate whether untimely first-line benzodiazepine treatment is associated with unfavorable short-term outcomes.
Design, setting, and participants: This multicenter, observational, prospective cohort study included 218 pediatric patients admitted between June 1, 2011, and July 7, 2016, into the 11 tertiary hospitals in the United States within the Pediatric Status Epilepticus Research Group. Patients, ranging in age from 1 month to 21 years, with refractory convulsive status epilepticus (RCSE) that did not stop after the administration of at least 2 antiseizure medications were included. Patients were divided into 2 cohorts: those who received the first-line benzodiazepine treatment in less than 10 minutes and those who received it 10 or more minutes after seizure onset (untimely). Data were collected and analyzed from June 1, 2011, to July 7, 2016.
Main outcomes and measures: The primary outcome was death during the related hospital admission. The secondary outcome was the need for continuous infusion for seizure termination. Multivariate analysis of mortality controlled for structural cause, febrile RCSE, age, and previous neurological history (including previous RCSE events). Use of continuous infusions was additionally adjusted for generalized RCSE, continuous RCSE, and 5 or more administrations of antiseizure medication.
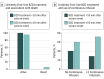
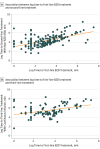
Results: A total of 218 patients were included, among whom 116 (53.2%) were male and the median (interquartile range) age was 4.0 (1.2-9.6) years. The RCSE started in the prehospital setting for 139 patients (63.8%). Seventy-four patients (33.9%) received their first-line benzodiazepine treatment in less than 10 minutes, and 144 (66.1%) received untimely first-line benzodiazepine treatment. Multivariate analysis showed that patients who received untimely first-line benzodiazepine treatment had higher odds of death (adjusted odds ratio [AOR], 11.0; 95% CI, 1.43 to ∞; P = .02), had greater odds of receiving continuous infusion (AOR, 1.8; 95% CI, 1.01-3.36; P = .047), had longer convulsive seizure duration (AOR, 2.6; 95% CI, 1.38-4.88; P = .003), and had more frequent hypotension (AOR 2.3; 95% CI, 1.16-4.63; P = .02). In addition, the timing of the first-line benzodiazepine treatment was correlated with the timing of the second-line (95% CI, 0.64-0.95; P < .001) and third-line antiseizure medications (95% CI, 0.25-0.78; P < .001).
Conclusions and relevance: Among pediatric patients with RCSE, an untimely first-line benzodiazepine treatment is independently associated with a higher frequency of death, use of continuous infusions, longer convulsion duration, and more frequent hypotension. Results of this study raise the question as to whether poor outcomes could, in part, be prevented by earlier administration of treatment.
Conflict of interest statement
Figures

Comment in
-
Time May Be of the Essence in the Treatment of Pediatric Patients With Refractory Convulsive Status Epilepticus.JAMA Neurol. 2018 Apr 1;75(4):402-403. doi: 10.1001/jamaneurol.2017.4028. JAMA Neurol. 2018. PMID: 29356824 No abstract available.
Similar articles
-
Automated identification and quality measurement for pediatric convulsive status epilepticus.Epilepsia. 2021 Feb;62(2):337-346. doi: 10.1111/epi.16795. Epub 2020 Dec 20. Epilepsia. 2021. PMID: 33341928
-
Levetiracetam as an alternative to phenytoin for second-line emergency treatment of children with convulsive status epilepticus: the EcLiPSE RCT.Health Technol Assess. 2020 Nov;24(58):1-96. doi: 10.3310/hta24580. Health Technol Assess. 2020. PMID: 33190679 Free PMC article. Clinical Trial.
-
Benzodiazepine administration patterns before escalation to second-line medications in pediatric refractory convulsive status epilepticus.Epilepsia. 2021 Nov;62(11):2766-2777. doi: 10.1111/epi.17043. Epub 2021 Aug 21. Epilepsia. 2021. PMID: 34418087 Free PMC article.
-
[Management of convulsive status epilepticus in infants and children].Rev Neurol (Paris). 2009 Apr;165(4):390-7. doi: 10.1016/j.neurol.2008.11.009. Epub 2009 Mar 4. Rev Neurol (Paris). 2009. PMID: 19264335 Review. French.
-
Gaps and opportunities in refractory status epilepticus research in children: a multi-center approach by the Pediatric Status Epilepticus Research Group (pSERG).Seizure. 2014 Feb;23(2):87-97. doi: 10.1016/j.seizure.2013.10.004. Epub 2013 Oct 16. Seizure. 2014. PMID: 24183923 Free PMC article. Review.
Cited by
-
Timing matters: there are significant differences in short-term outcomes between two time points of status epilepticus.BMC Neurol. 2022 Sep 14;22(1):348. doi: 10.1186/s12883-022-02868-y. BMC Neurol. 2022. PMID: 36104657 Free PMC article.
-
Why ketamine.Epilepsy Behav. 2023 Apr;141:109066. doi: 10.1016/j.yebeh.2022.109066. Epub 2023 Jan 4. Epilepsy Behav. 2023. PMID: 36609129 Free PMC article. Review.
-
A Theoretical Paradigm for Evaluating Risk-Benefit of Status Epilepticus Treatment.J Clin Neurophysiol. 2020 Sep;37(5):385-392. doi: 10.1097/WNP.0000000000000753. J Clin Neurophysiol. 2020. PMID: 32890059 Free PMC article. Review.
-
Clinician-Reported Physical and Cognitive Impairments After Convulsive Status Epilepticus: Post Hoc Study of a Randomized Controlled Trial.Neurocrit Care. 2024 Apr;40(2):495-505. doi: 10.1007/s12028-023-01758-6. Epub 2023 Jun 7. Neurocrit Care. 2024. PMID: 37286846 Clinical Trial.
-
Excitatory GABAergic signalling is associated with benzodiazepine resistance in status epilepticus.Brain. 2019 Nov 1;142(11):3482-3501. doi: 10.1093/brain/awz283. Brain. 2019. PMID: 31553050 Free PMC article.
References
-
- Commission on Classification and Terminology of the International League Against Epilepsy Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22(4):489-501. - PubMed
-
- Chin RF, Neville BG, Peckham C, Bedford H, Wade A, Scott RC; NLSTEPSS Collaborative Group . Incidence, cause, and short-term outcome of convulsive status epilepticus in childhood: prospective population-based study. Lancet. 2006;368(9531):222-229. - PubMed
-
- DeLorenzo RJ, Hauser WA, Towne AR, et al. . A prospective, population-based epidemiologic study of status epilepticus in Richmond, Virginia. Neurology. 1996;46(4):1029-1035. - PubMed
-
- Hesdorffer DC, Logroscino G, Cascino G, Annegers JF, Hauser WA. Incidence of status epilepticus in Rochester, Minnesota, 1965-1984. Neurology. 1998;50(3):735-741. - PubMed
-
- Chin RF, Neville BG, Scott RC. A systematic review of the epidemiology of status epilepticus. Eur J Neurol. 2004;11(12):800-810. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

