The Temporal Regulation of S Phase Proteins During G1
- PMID: 29357066
- PMCID: PMC5909198
- DOI: 10.1007/978-981-10-6955-0_16
The Temporal Regulation of S Phase Proteins During G1
Abstract
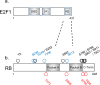
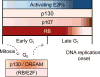
Successful DNA replication requires intimate coordination with cell-cycle progression. Prior to DNA replication initiation in S phase, a series of essential preparatory events in G1 phase ensures timely, complete, and precise genome duplication. Among the essential molecular processes are regulated transcriptional upregulation of genes that encode replication proteins, appropriate post-transcriptional control of replication factor abundance and activity, and assembly of DNA-loaded protein complexes to license replication origins. In this chapter we describe these critical G1 events necessary for DNA replication and their regulation in the context of both cell-cycle entry and cell-cycle progression.
Keywords: APC/C; CDK; Cell cycle; Cyclin; DREAM complex; E2F; Origin licensing; Quiescence; RB.
Conflict of interest statement
Conflicts of interest: The authors have no conflicts of interest.
Figures





References
-
- Avni D, Yang H, Martelli F, Hofmann F, ElShamy WM, Ganesan S, Scully R, Livingston DM. Active localization of the retinoblastoma protein in chromatin and its response to S phase DNA damage. Mol Cell. 2003;12(3):735–746. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

