Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation
- PMID: 29357120
- PMCID: PMC6491341
- DOI: 10.1002/14651858.CD011551.pub2
Magnetic resonance perfusion for differentiating low-grade from high-grade gliomas at first presentation
Abstract
Background: Gliomas are the most common primary brain tumour. They are graded using the WHO classification system, with Grade II-IV astrocytomas, oligodendrogliomas and oligoastrocytomas. Low-grade gliomas (LGGs) are WHO Grade II infiltrative brain tumours that typically appear solid and non-enhancing on magnetic resonance imaging (MRI) scans. People with LGG often have little or no neurologic deficit, so may opt for a watch-and-wait-approach over surgical resection, radiotherapy or both, as surgery can result in early neurologic disability. Occasionally, high-grade gliomas (HGGs, WHO Grade III and IV) may have the same MRI appearance as LGGs. Taking a watch-and-wait approach could be detrimental for the patient if the tumour progresses quickly. Advanced imaging techniques are increasingly used in clinical practice to predict the grade of the tumour and to aid clinical decision of when to intervene surgically. One such advanced imaging technique is magnetic resonance (MR) perfusion, which detects abnormal haemodynamic changes related to increased angiogenesis and vascular permeability, or "leakiness" that occur with aggressive tumour histology. These are reflected by changes in cerebral blood volume (CBV) expressed as rCBV (ratio of tumoural CBV to normal appearing white matter CBV) and permeability, measured by Ktrans.
Objectives: To determine the diagnostic test accuracy of MR perfusion for identifying patients with primary solid and non-enhancing LGGs (WHO Grade II) at first presentation in children and adults. In performing the quantitative analysis for this review, patients with LGGs were considered disease positive while patients with HGGs were considered disease negative.To determine what clinical features and methodological features affect the accuracy of MR perfusion.
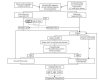
Search methods: Our search strategy used two concepts: (1) glioma and the various histologies of interest, and (2) MR perfusion. We used structured search strategies appropriate for each database searched, which included: MEDLINE (Ovid SP), Embase (Ovid SP), and Web of Science Core Collection (Science Citation Index Expanded and Conference Proceedings Citation Index). The most recent search for this review was run on 9 November 2016.We also identified 'grey literature' from online records of conference proceedings from the American College of Radiology, European Society of Radiology, American Society of Neuroradiology and European Society of Neuroradiology in the last 20 years.
Selection criteria: The titles and abstracts from the search results were screened to obtain full-text articles for inclusion or exclusion. We contacted authors to clarify or obtain missing/unpublished data.We included cross-sectional studies that performed dynamic susceptibility (DSC) or dynamic contrast-enhanced (DCE) MR perfusion or both of untreated LGGs and HGGs, and where rCBV and/or Ktrans values were reported. We selected participants with solid and non-enhancing gliomas who underwent MR perfusion within two months prior to histological confirmation. We excluded studies on participants who received radiation or chemotherapy before MR perfusion, or those without histologic confirmation.
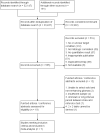
Data collection and analysis: Two review authors extracted information on study characteristics and data, and assessed the methodological quality using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool. We present a summary of the study characteristics and QUADAS-2 results, and rate studies as good quality when they have low risk of bias in the domains of reference standard of tissue diagnosis and flow and timing between MR perfusion and tissue diagnosis.In the quantitative analysis, LGGs were considered disease positive, while HGGs were disease negative. The sensitivity refers to the proportion of LGGs detected by MR perfusion, and specificity as the proportion of detected HGGs. We constructed two-by-two tables with true positives and false negatives as the number of correctly and incorrectly diagnosed LGG, respectively, while true negatives and false positives are the number of correctly and incorrectly diagnosed HGG, respectively.Meta-analysis was performed on studies with two-by-two tables, with further sensitivity analysis using good quality studies. Limited data precluded regression analysis to explore heterogeneity but subgroup analysis was performed on tumour histology groups.
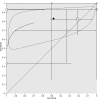
Main results: Seven studies with small sample sizes (4 to 48) met our inclusion criteria. These were mostly conducted in university hospitals and mostly recruited adult patients. All studies performed DSC MR perfusion and described heterogeneous acquisition and post-processing methods. Only one study performed DCE MR perfusion, precluding quantitative analysis.Using patient-level data allowed selection of individual participants relevant to the review, with generally low risks of bias for the participant selection, reference standard and flow and timing domains. Most studies did not use a pre-specified threshold, which was considered a significant source of bias, however this did not affect quantitative analysis as we adopted a common rCBV threshold of 1.75 for the review. Concerns regarding applicability were low.From published and unpublished data, 115 participants were selected and included in the meta-analysis. Average rCBV (range) of 83 LGGs and 32 HGGs were 1.29 (0.01 to 5.10) and 1.89 (0.30 to 6.51), respectively. Using the widely accepted rCBV threshold of <1.75 to differentiate LGG from HGG, the summary sensitivity/specificity estimates were 0.83 (95% CI 0.66 to 0.93)/0.48 (95% CI 0.09 to 0.90). Sensitivity analysis using five good quality studies yielded sensitivity/specificity of 0.80 (95% CI 0.61 to 0.91)/0.67 (95% CI 0.07 to 0.98). Subgroup analysis for tumour histology showed sensitivity/specificity of 0.92 (95% CI 0.55 to 0.99)/0.42 (95% CI 0.02 to 0.95) in astrocytomas (6 studies, 55 participants) and 0.77 (95% CI 0.46 to 0.93)/0.53 (95% CI 0.14 to 0.88) in oligodendrogliomas+oligoastrocytomas (6 studies, 56 participants). Data were too sparse to investigate any differences across subgroups.
Authors' conclusions: The limited available evidence precludes reliable estimation of the performance of DSC MR perfusion-derived rCBV for the identification of grade in untreated solid and non-enhancing LGG from that of HGG. Pooled data yielded a wide range of estimates for both sensitivity (range 66% to 93% for detection of LGGs) and specificity (range 9% to 90% for detection of HGGs). Other clinical and methodological features affecting accuracy of the technique could not be determined from the limited data. A larger sample size of both LGG and HGG, preferably using a standardised scanning approach and with an updated reference standard incorporating molecular profiles, is required for a definite conclusion.
Conflict of interest statement
Jill M Abrigo ‐ Has no competing interest to declare Wilson Wai San Tam ‐ None known Dan Fountain ‐ None known Michael G Hart ‐ None Known Eric Ka Chai Law ‐ None Known Joey SW Kwong ‐ None known James M Provenzale ‐ Reports that he holds consultancies, industry‐sponsored lectures and grants but avows that they have no relationship to the research topic being studied herein.
Figures




Update of
- doi: 10.1002/14651858.CD011551
Similar articles
-
Regional cerebral blood flow single photon emission computed tomography for detection of Frontotemporal dementia in people with suspected dementia.Cochrane Database Syst Rev. 2015 Jun 23;2015(6):CD010896. doi: 10.1002/14651858.CD010896.pub2. Cochrane Database Syst Rev. 2015. PMID: 26102272 Free PMC article.
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
-
Diagnostic test accuracy and cost-effectiveness of tests for codeletion of chromosomal arms 1p and 19q in people with glioma.Cochrane Database Syst Rev. 2022 Mar 2;3(3):CD013387. doi: 10.1002/14651858.CD013387.pub2. Cochrane Database Syst Rev. 2022. PMID: 35233774 Free PMC article.
-
Doppler trans-thoracic echocardiography for detection of pulmonary hypertension in adults.Cochrane Database Syst Rev. 2022 May 9;5(5):CD012809. doi: 10.1002/14651858.CD012809.pub2. Cochrane Database Syst Rev. 2022. PMID: 35532166 Free PMC article.
-
Intraoperative frozen section analysis for the diagnosis of early stage ovarian cancer in suspicious pelvic masses.Cochrane Database Syst Rev. 2016 Mar 1;3(3):CD010360. doi: 10.1002/14651858.CD010360.pub2. Cochrane Database Syst Rev. 2016. PMID: 26930463 Free PMC article.
Cited by
-
Review of tracer kinetic models in evaluation of gliomas using dynamic contrast-enhanced imaging.Front Oncol. 2024 Jun 14;14:1380793. doi: 10.3389/fonc.2024.1380793. eCollection 2024. Front Oncol. 2024. PMID: 38947892 Free PMC article. Review.
-
Advanced Diagnosis of Glioma by Using Emerging Magnetic Resonance Sequences.Front Oncol. 2021 Aug 5;11:694498. doi: 10.3389/fonc.2021.694498. eCollection 2021. Front Oncol. 2021. PMID: 34422648 Free PMC article. Review.
-
MRI biomarkers in neuro-oncology.Nat Rev Neurol. 2021 Aug;17(8):486-500. doi: 10.1038/s41582-021-00510-y. Epub 2021 Jun 20. Nat Rev Neurol. 2021. PMID: 34149051 Review.
-
Hemodynamic Imaging in Cerebral Diffuse Glioma-Part A: Concept, Differential Diagnosis and Tumor Grading.Cancers (Basel). 2022 Mar 10;14(6):1432. doi: 10.3390/cancers14061432. Cancers (Basel). 2022. PMID: 35326580 Free PMC article. Review.
-
The surgical perspective in precision treatment of diffuse gliomas.Onco Targets Ther. 2019 Feb 22;12:1497-1508. doi: 10.2147/OTT.S174316. eCollection 2019. Onco Targets Ther. 2019. PMID: 30863116 Free PMC article. Review.
References
References to studies included in this review
Cuccarini 2016 {published and unpublished data}
Falk 2014 {published and unpublished data}
-
- Falk A, Fahlström M, Rostrup E, Berntsson S, Zetterling M, Morell A, et al. Discrimination between glioma grades II and III in suspected low‐grade gliomas using dynamic contrast‐enhanced and dynamic susceptibility contrast perfusion MR imaging: a histogram analysis approach. Neuroradiology 2014;56:1031‐8. [DOI: 10.1007/s00234-014-1426-z] - DOI - PubMed
Guzman de Villoria 2014 {published and unpublished data}
Koob 2016 {published and unpublished data}
Kudo 2016 {published and unpublished data}
-
- Kudo K, Uwano I, Hirai T, Murakami R, Nakamura H, Fujima N, et al. Comparison of different post‐processing algorithms for dynamic susceptibility contrast perfusion imaging of cerebral gliomas. Magnetic Resonance in Medical Sciences : MRMS 2016 [Epub ahead of print];16(2):129‐36. [DOI: 10.2463/mrms.mp.2016-0036] - DOI - PMC - PubMed
Maia 2004 {published and unpublished data}
-
- Maia ACM, Malheiros SMF, Rocha AJ, Stávale JN, Guimaraes IF, Borges LRR, et al. Stereotactic biopsy guidance in adults with supratentorial nonenhancing gliomas: role of perfusion‐weighted magnetic resonance imaging. Journal of Neurosurgery 2004;101:970‐6. [DOI: 10.3171/jns.2004.101.6.0970] - DOI - PubMed
Yang 2002 {published data only}
-
- Yang D, Korogi Y, Sugahara T, Kitajima M, Shigematsu Y, Liang L, et al. Cerebral gliomas: prospective comparison of multivoxel 2D chemical‐shift imaging protonMR spectroscopy, echoplanar perfusionand diffusion‐weighted MRI. Neuroradiology 2002;44:656‐66. [DOI: 10.1007/s00234-002-0816-9] - DOI - PubMed
References to studies excluded from this review
Fan 2006 {published data only}
Gaudino 2010 {published and unpublished data}
-
- Gaudino S, Lorusso VS, Caulo M, Tartaro A, Tartaglione T, Lella G, et al. Multimodal MRI and overall diagnostic accuracy in non‐enhancing brain gliomas. Neuroradiology Journal. 2010; Vol. 23:145.
Law 2003 {published data only}
-
- Law M, Yang S, Wang H, Babb JS, Johnson G, Cha S, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR Imaging and Proton MR spectroscopic imaging compared with conventional MR imaging. AJNR. American Journal of Neuroradiology 2003;24(10):1989‐98. [PUBMED: 14625221] - PMC - PubMed
Lev 2004 {published data only}
-
- Lev MH, Ozsunar Y, Henson JW, Rasheed AA, Barest GD, Harsh GR 4th, et al. Glial tumor grading and outcome prediction using dynamic spin‐echo MR susceptibility mapping compared with conventional contrast‐enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas. AJNR. American Journal of Neuroradiology 2004;25(2):214‐21. [PUBMED: 14970020] - PMC - PubMed
Liu 2011 {published data only}
Morita 2010 {published data only}
Rollin 2006 {published data only}
Romano 2011 {published data only}
-
- Romano A, Coppola V, Cipriani V, Bonamini M, Trasimeni G, Fantozzi LM, et al. Role of fractional anisotropy and RCBV in differential diagnosis between low grade oligodendrogliomas and anaplastic astrocitomas. Neuroradiology. 2011; Vol. 53:S36‐S37.
Sahin 2013 {published data only}
-
- Sahin N, Melhem ER, Wang S, Krejza J, Poptani H, Chawla S, et al. Advanced MR imaging techniques in the evaluation of nonenhancing gliomas: perfusion‐weighted imaging compared with proton magnetic resonance spectroscopy and tumor grade. Neuroradiology 2013;26(5):531–41. [DOI: 10.1177/197140091302600506] - DOI - PMC - PubMed
Senturk 2009 {published data only}
-
- Sentürk S, Oğuz KK, Cila A. Dynamic contrast‐enhanced susceptibility‐weighted perfusion imaging of intracranial tumors: a study using a 3T MR scanner. Diagnostic and Interventional Radiology (Ankara, Turkey) 2009;15(1):3‐12. [PUBMED: 19263367] - PubMed
Sugahara 1998 {published data only}
-
- Sugahara T, Korogi Y, Kochi M, Ikushima I, Hirai T, Okuda T, et al. Correlation of MR imaging‐determined cerebral blood volume maps with histologic and angiographic determination of vascularity of gliomas. AJR. American Journal of Roentgenology 1998;171:1479‐86. [DOI: 10.2214/ajr.171.6.9843274] - DOI - PubMed
Additional references
Afra 1999
-
- Afra D, Osztie E, Sipos L, Vitanovics D. Preoperative history and postoperative survival of supratentorial low‐grade astrocytomas. British Journal of Neurosurgery 1999;13(3):299‐305. [PUBMED: 10562842] - PubMed
Al‐Okaili 2006
Anzalone 2017
-
- Anzalone N, Castellano A, Cadioli M, Conte GM, Cuccarini V, Bizzi A, et al. A multi‐center, standardized assessment of dynamic contrast‐enhanced and dynamic susceptibility contrast MRI in grading gliomas. Radiology 2017 (in press). - PubMed
Barker 1997
-
- Barker FG, 2nd, Chang SM, Huhn SL, Davis RL, Gutin PH, McDermott MW, et al. Age and the risk of anaplasia in magnetic resonance‐nonenhancing supratentorial cerebral tumors. Cancer 1997;80(5):936‐41. [PUBMED: 9307194] - PubMed
Bello 2010
Bernstein 1994
-
- Bernstein M, Guha A. Biopsy of low‐grade astrocytomas. Journal of Neurosurgery 1994;80(4):776‐7. - PubMed
Boissonneau 2017
Brat 2008
Brazzelli 2009
Claus 2006
Cohen‐Gadol 2004
Crocetti 2012
Deeks 2005
Dietrich 2017
-
- Dietrich J. Clinical presentation, initial surgical approach, and prognosis of high‐grade gliomas. www.uptodate.com (Accessed on December 17, 2017).
Dolecek 2012
Duffau 2005
-
- Duffau H, Lopes M, Arthuis F, Bitar A, Sichez JP, Effenterre R, et al. Contribution of intraoperative electrical stimulations in surgery of low grade gliomas: a comparative study between two series without (1985‐96) and with (1996‐2003) functional mapping in the same institution. Journal of Neurology, Neurosurgery & Psychiatry 2005;76(6):845‐51. [DOI: 10.1136/jnnp.2004.048520] - DOI - PMC - PubMed
Essig 2013
Heiss 2011
Irwig 1995
-
- Irwig L, Macaskill P, Glasziou P, Fahey M. Meta‐analytic methods for diagnostic test accuracy. Journal of Clinical Epidemiology 1995;48(1):119‐30. [PUBMED: 7853038] - PubMed
Jakola 2012
Jakola 2017
Kondziolka 1993
Kreth 2001
-
- Kreth FW, Muacevic A, Medele R, Bise K, Meyer T, Reulen HJ. The risk of haemorrhage after image guided stereotactic biopsy of intra‐axial brain tumours—a prospective study. Acta Neurochirurgica (Wien) 2001;143:539–45. [PUBMED: 11534670] - PubMed
Louis 2007
Louis 2016
Macaskill 2010
-
- Macaskill P, Gatsonis C, Deeks JJ, Harbord RM, Takwoingi Y. Chapter 10: Analysing and Presenting Results. In: Deeks JJ, Bossuyt PM, Gatsonis C editor(s). Cochrane Handbook for Systematic Reviews of Diagnostic Test Accuracy Ver 1.0, available from: http://srdta.cochrane.org/. The Cochrane Collaboration, 2010.
Muragaki 2008
NCCN Guidelines 2016
-
- National Comprehensive Cancer Network. Central Nervous System Cancers (Version 1.2016). https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf Accessed July 2017.
Nicolato 1995
-
- Nicolato A, Gerosa MA, Fina P, Iuzzolino P, Giorguitti F, Bricolo A. Prognostic factors in low‐grade supratentorial astrocytomas: a uni‐multivariate statistical analysis in 76 surgically treated adult patients. Surgical Neurology 1995;44:208‐23. [PUBMED: 8545771] - PubMed
Ohgaki 2005
Pallud 2009
Partlett 2016
-
- Partlett C, Takwoingi Y. Meta‐analysis of test accuracy studies in R: a summary of user‐written programs and step‐by‐step guide to using glmer. Version 1.0. Available from: http://methods.cochrane.org/sdt/ August 2016.
Paugh 2010
Piepmeier 1996
-
- Piepmeier J, Christopher S, Spencer D, Byrne T, Kim J, Knisel JP, et al. Variations in the natural history and survival of patients with supratentorial low‐grade astrocytomas. Neurosurgery 1996;38(5):872‐8. [PUBMED: 8727811] - PubMed
Piepmeier 2009
Pignatti 2002
Provenzale 2006
R Core team 2013
-
- R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R‐project.org/ 2013.
Recht 1992
Rees 2002
Reijneveld 2001
-
- Reijneveld JC, Sitskoorn MM, Klein M, Nuyen J, Taphoorn MJ. Cognitive status and quality of life in patients with suspected versus proven low‐grade gliomas. Neurology 2001;56(5):618‐23. [PUBMED: 11245713] - PubMed
Reitsma 2005
Review Manager 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). The Nordic Cochrane Centre, The Cochrane Collaboration,. Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Scott 2002
-
- Scott JN, Brasher PM, Sevick RJ, Rewcastle NB, Forsyth PA. How often are nonenhancing supratentorial gliomas malignant? A population study. Neurology 2002;59(6):947‐9. [PUBMED: 12297589] - PubMed
Soffietti 2010
van den Bent 2005
-
- Bent MJ, Afra D, Witte O, Ben Hassel M, Schraub S, Hoang‐Xuan K, et al. Long‐term efficacy of early versus delayed radiotherapy for low‐grade astrocytoma and oligodendroglioma in adults: the EORTC 22845 randomised trial. Lancet 2005;366(9490):985‐90. [DOI: 10.1016/S0140-6736(05)67070-5] - DOI - PubMed
Welker 2015
-
- Welker K, Boxerman J, Kalnin A, Kaufmann T, Shiroishi M, Wintermark M, American Society of Functional Neuroradiology MR Perfusion Standards and Practice Subcommittee of the ASFNR Clinical Practice Committee. ASFNR recommendations for clinical performance of MR dynamic susceptibility contrast perfusion imaging of the brain. AJNR. American Journal of Neuroradiology 2015;36(6):E41‐51. [DOI: 10.3174/ajnr.A4341] - DOI - PMC - PubMed
Weller 2017
-
- Weller M, Bent M, Tonn JC, Stupp R, Preusser M, Cohen‐Jonathan‐Moyal E, et al. European Association for Neuro‐Oncology (EANO) Task Force on Gliomas. European Association for Neuro‐Oncology (EANO) guideline on the diagnosis and treatment of adult astrocytic and oligodendroglial gliomas. Lancet Oncology 2017;6:e315‐e329. [DOI: 10.1016/S1470-2045(17)30194-8] - DOI - PubMed
Whiting 2011
Whittle 2004
References to other published versions of this review
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

