Apelinergic System Structure and Function
- PMID: 29357134
- PMCID: PMC5821487
- DOI: 10.1002/cphy.c170028
Apelinergic System Structure and Function
Abstract
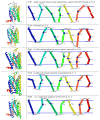
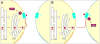
Apelin and apela (ELABELA/ELA/Toddler) are two peptide ligands for a class A G-protein-coupled receptor named the apelin receptor (AR/APJ/APLNR). Ligand-AR interactions have been implicated in regulation of the adipoinsular axis, cardiovascular system, and central nervous system alongside pathological processes. Each ligand may be processed into a variety of bioactive isoforms endogenously, with apelin ranging from 13 to 55 amino acids and apela from 11 to 32, typically being cleaved C-terminal to dibasic proprotein convertase cleavage sites. The C-terminal region of the respective precursor protein is retained and is responsible for receptor binding and subsequent activation. Interestingly, both apelin and apela exhibit isoform-dependent variability in potency and efficacy under various physiological and pathological conditions, but most studies focus on a single isoform. Biophysical behavior and structural properties of apelin and apela isoforms show strong correlations with functional studies, with key motifs now well determined for apelin. Unlike its ligands, the AR has been relatively difficult to characterize by biophysical techniques, with most characterization to date being focused on effects of mutagenesis. This situation may improve following a recently reported AR crystal structure, but there are still barriers to overcome in terms of comprehensive biophysical study. In this review, we summarize the three components of the apelinergic system in terms of structure-function correlation, with a particular focus on isoform-dependent properties, underlining the potential for regulation of the system through multiple endogenous ligands and isoforms, isoform-dependent pharmacological properties, and biological membrane-mediated receptor interaction. © 2018 American Physiological Society. Compr Physiol 8:407-450, 2018.
Copyright © 2018 John Wiley & Sons, Inc.
Figures






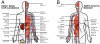

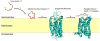



References
-
- Adam F, Khatib AM, Lopez JJ, Vatier C, Turpin S, Muscat A, Soulet F, Aries A, Jardin I, Bobe R, Stepanian A, de Prost D, Dray C, Rosado JA, Valet P, Feve B, Siegfried G. Apelin: an antithrombotic factor that inhibits platelet function. Blood. 2016;127:908–920. - PubMed
-
- Akcilar R, Turgut S, Caner V, Akcilar A, Ayada C, Elmas L, Ozcan TO. Apelin effects on blood pressure and RAS in DOCA-salt-induced hypertensive rats. Clin Exp Hypertens. 2013;35:550–557. - PubMed
-
- Akcilar R, Turgut S, Caner V, Akcilar A, Ayada C, Elmas L, Ozcan TO. The effects of apelin treatment on a rat model of type 2 diabetes. Adv Med Sci. 2015;60:94–100. - PubMed
-
- Andersen CU, Markvardsen LH, Hilberg O, Simonsen U. Pulmonary apelin levels and effects in rats with hypoxic pulmonary hypertension. Respir Med. 2009;103:1663–1671. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

