Candidate biomarkers for the diagnosis and prognosis of drug-induced liver injury: An international collaborative effort
- PMID: 29357190
- PMCID: PMC6054900
- DOI: 10.1002/hep.29802
Candidate biomarkers for the diagnosis and prognosis of drug-induced liver injury: An international collaborative effort
Abstract
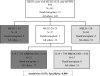
Current blood biomarkers are suboptimal in detecting drug-induced liver injury (DILI) and predicting its outcome. We sought to characterize the natural variabilty and performance characteristics of 14 promising DILI biomarker candidates. Serum or plasma from multiple cohorts of healthy volunteers (n = 192 and n = 81), subjects who safely took potentially hepatotoxic drugs without adverse effects (n = 55 and n = 92) and DILI patients (n = 98, n = 28, and n = 143) were assayed for microRNA-122 (miR-122), glutamate dehydrogenase (GLDH), total cytokeratin 18 (K18), caspase cleaved K18, glutathione S-transferase α, alpha-fetoprotein, arginase-1, osteopontin (OPN), sorbitol dehydrogenase, fatty acid binding protein, cadherin-5, macrophage colony-stimulating factor receptor (MCSFR), paraoxonase 1 (normalized to prothrombin protein), and leukocyte cell-derived chemotaxin-2. Most candidate biomarkers were significantly altered in DILI cases compared with healthy volunteers. GLDH correlated more closely with gold standard alanine aminotransferase than miR-122, and there was a surprisingly wide inter- and intra-individual variability of miR-122 levels among healthy volunteers. Serum K18, OPN, and MCSFR levels were most strongly associated with liver-related death or transplantation within 6 months of DILI onset. Prediction of prognosis among DILI patients using the Model for End-Stage Liver Disease was improved by incorporation of K18 and MCSFR levels. Conclusion: GLDH appears to be more useful than miR-122 in identifying DILI patients, and K18, OPN, and MCSFR are promising candidates for prediction of prognosis during an acute DILI event. Serial assessment of these biomarkers in large prospective studies will help further delineate their role in DILI diagnosis and management.
© 2018 by the American Association for the Study of Liver Diseases.
Figures

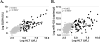

Comment in
-
New biomarkers for drug-induced liver injury.Hepatology. 2018 Jun;67(6):2480-2481. doi: 10.1002/hep.29865. Epub 2018 Apr 27. Hepatology. 2018. PMID: 29500900 No abstract available.
-
Reply.Hepatology. 2018 Jun;67(6):2481-2482. doi: 10.1002/hep.29863. Epub 2018 May 1. Hepatology. 2018. PMID: 29506324 No abstract available.
References
-
- Ostapowicz G, Fontana RJ, Schiodt FV, Larson A, Davern TJ, Han SH, et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann Intern Med. 2002;137:947–54. - PubMed
-
- Wei G, Bergquist A, Broome U, Lindgren S, Wallerstedt S, Almer S, et al. Acute liver failure in Sweden: etiology and outcome. J Intern Med. 2007;262:393–401. - PubMed
-
- Ruhl CE, Everhart JE. Determinants of the association of overweight with elevated serum alanine aminotransferase activity in the United States. Gastroenterology. 2003;124:71–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U01 DK065211/DK/NIDDK NIH HHS/United States
- U01 DK065238/DK/NIDDK NIH HHS/United States
- U01 DK065193/DK/NIDDK NIH HHS/United States
- U01 DK083023/DK/NIDDK NIH HHS/United States
- UL1 TR001420/TR/NCATS NIH HHS/United States
- U01 DK082992/DK/NIDDK NIH HHS/United States
- European Federation of Pharmaceutical Industries and Associations companies/International
- National Institute for Health Research Nottingham Digestive Diseases Biomedical Research Unit at the Nottingham University Hospitals National Health Service Trust/International
- P30 ES010126/ES/NIEHS NIH HHS/United States
- UL1 RR025761/RR/NCRR NIH HHS/United States
- FP7/2007-2013/European Union's Seventh Framework Programme/International
- U01 DK083027/DK/NIDDK NIH HHS/United States
- U01 DK083020/DK/NIDDK NIH HHS/United States
- University of Nottingham/International
- U01 DK065201/DK/NIDDK NIH HHS/United States
- U01 DK100928/DK/NIDDK NIH HHS/United States
- U01-DK083027/DK/NIDDK NIH HHS/United States
- U01 DK065176/DK/NIDDK NIH HHS/United States
- 115003/Innovative Medicines Initiative Joint Undertaking/International
- UL1 TR002240/TR/NCATS NIH HHS/United States
- UL1 RR025747/RR/NCRR NIH HHS/United States
- TL1 TR001107/TR/NCATS NIH HHS/United States
- UL1 RR024986/RR/NCRR NIH HHS/United States
- U01 DK065184/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

