Tamm-Horsfall protein/uromodulin deficiency elicits tubular compensatory responses leading to hypertension and hyperuricemia
- PMID: 29357410
- PMCID: PMC6032075
- DOI: 10.1152/ajprenal.00233.2017
Tamm-Horsfall protein/uromodulin deficiency elicits tubular compensatory responses leading to hypertension and hyperuricemia
Abstract
Expression of Tamm-Horsfall protein (THP or uromodulin) is highly restricted to the kidney thick ascending limb (TAL) of loop of Henle. Despite the unique location and recent association of THP gene mutations with hereditary uromodulin-associated kidney disease and THP single nucleotide polymorphisms with chronic kidney disease and hypertension, the physiological function(s) of THP and its pathological involvement remain incompletely understood. By studying age-dependent changes of THP knockout (KO) mice, we show here that young KO mice had significant salt and water wasting but were partially responsive to furosemide, due to decreased luminal translocation of Na-K-Cl cotransporter 2 (NKCC2) in the TAL. Aged THP KO mice were, however, markedly oliguric and unresponsive to furosemide, and their NKCC2 was localized primarily in the cytoplasm as evidenced by lipid raft floatation assay, cell fractionation, and confocal and immunoelectron microscopy. These aged KO mice responded to metolazone and acetazolamide, known to target distal and proximal tubules, respectively. They also had marked upregulation of renin in juxtaglomerular apparatus and serum, and they were hypertensive. Finally, the aged THP KO mice had significant upregulation of Na-coupled urate transporters Slc5a8 and Slc22a12 as well as sodium-hydrogen exchanger 3 (NHE3) in the proximal tubule and elevated serum uric acid and allantoin. Collectively, our results suggest that THP deficiency can cause progressive disturbances in renal functions via initially NKCC2 dysfunction and later compensatory responses, resulting in prolonged activation of the renin-angiotensin-aldosterone axis and hyperuricemia.
Keywords: NKCC2; Tamm-Horsfall protein; hyperuricemia; lipid rafts; thick ascending limb; uromodulin.
Figures
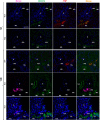
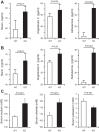
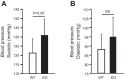
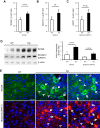


Comment in
-
Modulation of tubular solute reuptake in UMOD knockout mice.Am J Physiol Renal Physiol. 2018 Aug 1;315(2):F238-F240. doi: 10.1152/ajprenal.00080.2018. Epub 2018 Mar 7. Am J Physiol Renal Physiol. 2018. PMID: 29513073 No abstract available.
Similar articles
-
Uromodulin (Tamm-Horsfall protein): guardian of urinary and systemic homeostasis.Nephrol Dial Transplant. 2020 Jan 1;35(1):33-43. doi: 10.1093/ndt/gfy394. Nephrol Dial Transplant. 2020. PMID: 30649494 Free PMC article. Review.
-
Point mutation in D8C domain of Tamm-Horsfall protein/uromodulin in transgenic mice causes progressive renal damage and hyperuricemia.PLoS One. 2017 Nov 16;12(11):e0186769. doi: 10.1371/journal.pone.0186769. eCollection 2017. PLoS One. 2017. PMID: 29145399 Free PMC article.
-
Activation of the bumetanide-sensitive Na+,K+,2Cl- cotransporter (NKCC2) is facilitated by Tamm-Horsfall protein in a chloride-sensitive manner.J Biol Chem. 2011 Aug 26;286(34):30200-10. doi: 10.1074/jbc.M111.222968. Epub 2011 Jul 7. J Biol Chem. 2011. PMID: 21737451 Free PMC article.
-
Tamm-Horsfall protein translocates to the basolateral domain of thick ascending limbs, interstitium, and circulation during recovery from acute kidney injury.Am J Physiol Renal Physiol. 2013 Apr 15;304(8):F1066-75. doi: 10.1152/ajprenal.00543.2012. Epub 2013 Feb 6. Am J Physiol Renal Physiol. 2013. PMID: 23389456 Free PMC article.
-
Uromodulin biology.Nephrol Dial Transplant. 2024 Jun 28;39(7):1073-1087. doi: 10.1093/ndt/gfae008. Nephrol Dial Transplant. 2024. PMID: 38211973 Free PMC article. Review.
Cited by
-
Serum uromodulin is inversely associated with arterial hypertension and the vasoconstrictive prohormone CT-proET-1 in the population-based KORA F4 study.PLoS One. 2020 Aug 7;15(8):e0237364. doi: 10.1371/journal.pone.0237364. eCollection 2020. PLoS One. 2020. PMID: 32764816 Free PMC article.
-
Urinary Podocyte Excretion Predicts Urinary Protein Selectivity and Renal Prognosis.Int J Nephrol. 2022 Jun 29;2022:2702651. doi: 10.1155/2022/2702651. eCollection 2022. Int J Nephrol. 2022. PMID: 35866051 Free PMC article.
-
Associations of plasma uromodulin and genetic variants with blood pressure responses to dietary salt interventions.J Clin Hypertens (Greenwich). 2021 Oct;23(10):1897-1906. doi: 10.1111/jch.14347. Epub 2021 Aug 7. J Clin Hypertens (Greenwich). 2021. PMID: 34363725 Free PMC article.
-
Uromodulin (Tamm-Horsfall protein): guardian of urinary and systemic homeostasis.Nephrol Dial Transplant. 2020 Jan 1;35(1):33-43. doi: 10.1093/ndt/gfy394. Nephrol Dial Transplant. 2020. PMID: 30649494 Free PMC article. Review.
-
Evolving Concepts in Uromodulin Biology, Physiology, and Its Role in Disease: a Tale of Two Forms.Hypertension. 2022 Nov;79(11):2409-2418. doi: 10.1161/HYPERTENSIONAHA.122.18567. Epub 2022 Aug 12. Hypertension. 2022. PMID: 35959659 Free PMC article. Review.
References
-
- Bernascone I, Janas S, Ikehata M, Trudu M, Corbelli A, Schaeffer C, Rastaldi MP, Devuyst O, Rampoldi L. A transgenic mouse model for uromodulin-associated kidney diseases shows specific tubulo-interstitial damage, urinary concentrating defect and renal failure. Hum Mol Genet 19: 2998–3010, 2010. doi:10.1093/hmg/ddq205. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

