Neuraminidase activity mediates IL-6 production by activated lupus-prone mesangial cells
- PMID: 29357434
- PMCID: PMC5966761
- DOI: 10.1152/ajprenal.00421.2017
Neuraminidase activity mediates IL-6 production by activated lupus-prone mesangial cells
Abstract
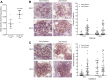
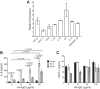
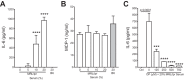
The development of nephritis is a leading cause of morbidity and mortality in lupus patients. Although the general pathophysiological progression of lupus nephritis is known, the molecular mediators and mechanisms are incompletely understood. Previously, we demonstrated that the glycosphingolipid (GSL) catabolic pathway is elevated in the kidneys of MRL/lpr lupus mice and human lupus patients with nephritis. Specifically, the activity of neuraminidase (NEU) and expression of Neu1, an enzyme in the GSL catabolic pathway is significantly increased. To better understand the role and mechanisms by which this pathway contributes to the progression of LN, we analyzed the expression and effects of NEU activity on the function of MRL/lpr lupus-prone mesangial cells (MCs). We demonstrate that NEU1 and NEU3 promote IL-6 production in MES13 MCs. Neu1 expression, NEU activity, and IL-6 production are significantly increased in stimulated primary MRL/lpr lupus-prone MCs, and blocking NEU activity inhibits IL-6 production. NEU1 and NEU3 expression overlaps IgG deposits in MCs in vitro and in renal sections from nephritic MRL/lpr mice. Together, our results suggest that NEU activity mediates IL-6 production in lupus-prone MCs possibly through an IgG-receptor complex signaling pathway.
Keywords: IL-6; lupus nephritis; mesangial cells; neuraminidase.
Figures




References
-
- Abdulkhalek S, Amith SR, Franchuk SL, Jayanth P, Guo M, Finlay T, Gilmour A, Guzzo C, Gee K, Beyaert R, Szewczuk MR. Neu1 sialidase and matrix metalloproteinase-9 cross-talk is essential for Toll-like receptor activation and cellular signaling. J Biol Chem 286: 36,532–36,549, 2011. doi: 10.1074/jbc.M111.237578. - DOI - PMC - PubMed
-
- Abdulkhalek S, Szewczuk MR. Neu1 sialidase and matrix metalloproteinase-9 cross-talk regulates nucleic acid-induced endosomal TOLL-like receptor-7 and -9 activation, cellular signaling and pro-inflammatory responses. Cell Signal 25: 2093–2105, 2013. doi: 10.1016/j.cellsig.2013.06.010. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

