Transmitter release site organization can predict synaptic function at the neuromuscular junction
- PMID: 29357458
- PMCID: PMC5966726
- DOI: 10.1152/jn.00168.2017
Transmitter release site organization can predict synaptic function at the neuromuscular junction
Abstract
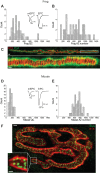
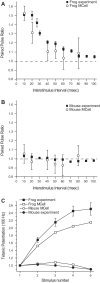
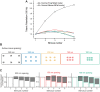
We have investigated the impact of transmitter release site (active zone; AZ) structure on synaptic function by physically rearranging the individual AZ elements in a previously published frog neuromuscular junction (NMJ) AZ model into the organization observed in a mouse NMJ AZ. We have used this strategy, purposefully without changing the properties of AZ elements between frog and mouse models (even though there are undoubtedly differences between frog and mouse AZ elements in vivo), to directly test how structure influences function at the level of an AZ. Despite a similarly ordered ion channel array substructure within both frog and mouse AZs, frog AZs are much longer and position docked vesicles in a different location relative to AZ ion channels. Physiologically, frog AZs have a lower probability of transmitter release compared with mouse AZs, and frog NMJs facilitate strongly during short stimulus trains in contrast with mouse NMJs that depress slightly. Using our computer modeling approach, we found that a simple rearrangement of the AZ building blocks of the frog model into a mouse AZ organization could recapitulate the physiological differences between these two synapses. These results highlight the importance of simple AZ protein organization to synaptic function. NEW & NOTEWORTHY A simple rearrangement of the basic building blocks in the frog neuromuscular junction model into a mouse transmitter release site configuration predicted the major physiological differences between these two synapses, suggesting that transmitter release site structure and organization is a strong predictor of function.
Keywords: active zone; neuromuscular junction; transmitter release.
Figures







References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

