HIV RNA Suppression during and after Pregnancy among Women in the HIV Outpatient Study, 1996 to 2015
- PMID: 29357772
- PMCID: PMC6748471
- DOI: 10.1177/2325957417752259
HIV RNA Suppression during and after Pregnancy among Women in the HIV Outpatient Study, 1996 to 2015
Abstract
Objective: To examine HIV viral suppression during/after pregnancy.
Design: Prospective observational cohort.
Methods: We identified pregnancies from 1996 to 2015. We examined HIV RNA viral load (VL), VL suppression (≤500 copies/mL), and antiretroviral therapy (ART) status at pregnancy start, end, and 6 months postpartum. We estimated risk ratios (RRs) and 95% confidence intervals (CIs) for VL nonsuppression.
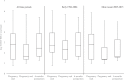
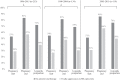
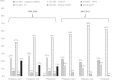
Results: Among 253 pregnancies analyzed, 34.8% of women exhibited VL suppression at pregnancy start, 60.1% at pregnancy end, and 42.7% at 6 months postpartum. Median VL (log10 copies/mL) was 2.80 (interquartile range [IQR]: 1.40-3.85) at pregnancy start, 1.70 (IQR: 1.40-2.82) at pregnancy end, and 2.30 (IQR: 1.40-3.86) at postpartum. Risk of postpartum VL nonsuppression was also lower among women on ART and with VL suppression at pregnancy end (versus those not; adjusted RR = 0.30, 95% CI: 0.17-0.53).
Conclusions: Maintaining VL suppression among US women remains a challenge, particularly during postpartum. Achieving VL suppression earlier during pregnancy benefits women subsequently.
Keywords: HIV; postpartum; pregnancy; viral suppression.
Conflict of interest statement
Figures
References
-
- Centers for Disease Control and Prevention. HIV Surveillance Report, 2014. Atlanta, GA: Centers for Disease Control and Prevention; 2015.
-
- Hamilton BE, Martin JA, Osterman MJ, Curtin SC, Matthews TJ. Births: final data for 2014. Natl Vital Stat Rep. 2015;64(12):1–64. - PubMed
-
- Whitmore SK, Zhang X, Taylor AW, Blair JM. Estimated number of infants born to HIV-infected women in the United States and five dependent areas, 2006. J Acquir Immune Defic Syndr. 2011;57(3):218–222. - PubMed
-
- Jamieson DJ, Clark J, Kourtis AP, et al. Recommendations for human immunodeficiency virus screening, prophylaxis, and treatment for pregnant women in the United States. Am J Obstetr Gynecol. 2007;197(3 suppl):S26–S32. - PubMed
-
- Connor EM, Sperling RS, Gelber R, et al. Reduction of maternal-infant transmission of human immunodeficiency virus type 1 with zidovudine treatment. N Engl J Med. 1994,331(18):1173–1180. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

