Caffeine is a risk factor for osteopenia of prematurity in preterm infants: a cohort study
- PMID: 29357829
- PMCID: PMC5776771
- DOI: 10.1186/s12887-017-0978-6
Caffeine is a risk factor for osteopenia of prematurity in preterm infants: a cohort study
Abstract
Background: Caffeine, the most commonly used medication in Neonatal Intensive Care Units, has calciuric and osteoclastogenic effects.
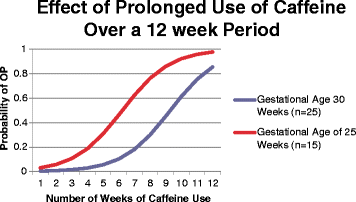
Methods: To examine the association between the cumulative dose and duration of therapy of caffeine and osteopenia of prematurity, a retrospective cohort study was conducted including premature infants less than 31 weeks and birth weight less than 1500 g. Osteopenia of prematurity was evaluated using chest X-rays on a biweekly basis over 12 weeks of hospitalization.
Results: The cohort included 109 infants. 51% had osteopenia of prematurity and 8% had spontaneous rib fractures. Using the generalized linear mixed model, caffeine dose and duration of caffeine therapy showed a strong association with osteopenia of prematurity. Steroids and vitamin D were also significantly correlated with osteopenia of prematurity while diuretic use did not show a statistically significant effect.
Conclusion: The cumulative dose and duration of therapy of caffeine, as well as steroid are associated with osteopenia of prematurity in this cohort. Future studies are needed to confirm these findings and determine the lowest dose of caffeine needed to treat effectively apnea of prematurity.
Keywords: Caffeine; Metabolic bone disease; Osteopenia of prematurity; Premature infants.
Conflict of interest statement
Ethics approval and consent to participate
The study was approved by the Health Research Ethics Board (HREB) at University of Manitoba number# H2013: 231, and the Health Sciences Center Research Impact Approval from the Health Science Center. Number# RI2013: 088. The included data were retrospective data from medical records and did not include any identifying information. Consent to participate is not applicable for this study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

