Practice and consensus-based strategies in diagnosing and managing systemic juvenile idiopathic arthritis in Germany
- PMID: 29357887
- PMCID: PMC5778670
- DOI: 10.1186/s12969-018-0224-2
Practice and consensus-based strategies in diagnosing and managing systemic juvenile idiopathic arthritis in Germany
Abstract
Background: Systemic juvenile idiopathic arthritis (SJIA) is an autoinflammatory disease associated with chronic arthritis. Early diagnosis and effective therapy of SJIA is desirable, so that complications are avoided. The PRO-KIND initiative of the German Society for Pediatric Rheumatology (GKJR) aims to define consensus-based strategies to harmonize diagnostic and therapeutic approaches in Germany.
Methods: We analyzed data on patients diagnosed with SJIA from 3 national registries in Germany. Subsequently, via online surveys and teleconferences among pediatric rheumatologists with a special expertise in the treatment of SJIA, we identified current diagnostic and treatment approaches in Germany. Those were harmonized via the formulation of statements and, supported by findings from a literature search. Finally, an in-person consensus conference using nominal group technique was held to further modify and consent the statements.
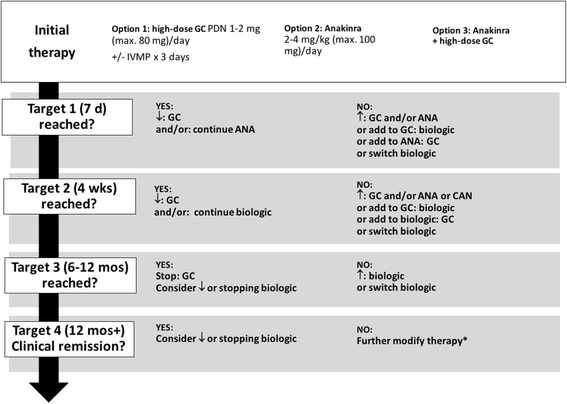
Results: Up to 50% of patients diagnosed with SJIA in Germany do not fulfill the International League of Associations for Rheumatology (ILAR) classification criteria, mostly due to the absence of chronic arthritis. Our findings suggest that chronic arthritis is not obligatory for the diagnosis and treatment of SJIA, allowing a diagnosis of probable SJIA. Malignant, infectious and hereditary autoinflammatory diseases should be considered before rendering a diagnosis of probable SJIA. There is substantial variability in the initial treatment of SJIA. Based on registry data, most patients initially receive systemic glucocorticoids, however, increasingly substituted or accompanied by biological agents, i.e. interleukin (IL)-1 and IL-6 blockade (up to 27.2% of patients). We identified preferred initial therapies for probable and definitive SJIA, including step-up patterns and treatment targets for the short-term (resolution of fever, decrease in C-reactive protein by 50% within 7 days), the mid-term (improvement in physician global and active joint count by at least 50% or a JADAS-10 score of maximally 5.4 within 4 weeks) and the long-term (glucocorticoid-free clinically inactive disease within 6 to 12 months), and an explicit treat-to-target strategy.
Conclusions: We developed consensus-based strategies regarding the diagnosis and treatment of probable or definitive SJIA in Germany.
Keywords: Biologics; Diagnosis; Glucocorticoids; Interleukin-1 blockade; Interleukin-6 blockade; Systemic juvenile idiopathic arthritis; Treat-to-target.
Conflict of interest statement
Ethics approval and consent to participate
The AID-Net was approved by the ethics committee of the physicians’ chamber Westfalen-Lippe and the University of Münster. Both ICON-JIA and the national pediatric rheumatology database were approved by the ethics committee of the Charité in Berlin.
Consent for publication
Not applicable.
Competing interests
Dr. Hinze has received consulting fees, speaking fees, and/or honoraria from Novartis (less than $10,000 each). Dr. Holzinger has received consulting fees, speaking fees, and/or honoraria from Novartis and Pfizer (less than $10,000 each) and research support from Pfizer. Dr. Lainka has received consulting fees, speaking fees, and/or honoraria from Novartis (less than $10,000 each) and research support from Sobi. Dr. Haas has received research support from Novartis and Pfizer. Dr. Kallinich has received consulting fees, speaking fees, and/or honoraria from Bristol-Myers-Squibb, CSL, Novartis and Roche (less than $10,000 each) and research support from Novartis. Dr. Rieber has received consulting fees, speaking fees, and/or honoraria from Novartis (less than $10,000 each) and research support from Novartis. Dr. Hufnagel has received research support from Novartis and Roche. Dr. Jansson has received consulting fees, speaking fees, and/or honoraria from Novartis (less than $10,000 each) and research support from Novartis. Dr. Hedrich has received consulting fees, speaking fees, and/or honoraria from Novartis and Roche (less than $10,000 each). Dr. Foeldvari has received consulting fees, speaking fees, and/or honoraria from Abbvie, Bayer, Chugai, Genentech, Medac, Novartis and Pfizer (less than $10,000 each). Dr. Hospach has received consulting fees, speaking fees, and/or honoraria from Chugai and Novartis (less than $10,000 each). Dr. Huppertz has received consulting fees, speaking fees, and/or honoraria from Chugai, Novartis, Pfizer and Roche (less than $10,000 each). Dr. Mönkemöller has received consulting fees, speaking fees, and/or honoraria from Novartis (less than $10,000 each). Dr. Weißbarth-Riedel has received consulting fees, speaking fees, and/or honoraria from Novartis (less than $10,000 each). Dr. Wittkowski has received consulting fees, speaking fees, and/or honoraria from Novartis (less than $10,000 each). Dr. Horneff has received honoraria from Abbvie, Novartis, Pfizer and Roche-Chugai (less than $10,000 each) and research support from Abbvie, Novartis, Pfizer and Roche-Chugai. Dr. Föll has received honoraria from Novartis, Pfizer, Roche-Chugai and Sobi (less than $10,000 each) and research support from Novartis and Pfizer.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- Nigrovic PA, Mannion M, Prince FH, Zeft A, Rabinovich CE, van Rossum MA, Cortis E, Pardeo M, Miettunen PM, Janow G, et al. Anakinra as first-line disease-modifying therapy in systemic juvenile idiopathic arthritis: report of forty-six patients from an international multicenter series. Arthritis Rheum. 2011;63(2):545–555. doi: 10.1002/art.30128. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

