eRegQual-an electronic health registry with interactive checklists and clinical decision support for improving quality of antenatal care: study protocol for a cluster randomized trial
- PMID: 29357912
- PMCID: PMC5778657
- DOI: 10.1186/s13063-017-2386-5
eRegQual-an electronic health registry with interactive checklists and clinical decision support for improving quality of antenatal care: study protocol for a cluster randomized trial
Abstract
Background: Health worker compliance with established best-practice clinical and public health guidelines may be enhanced by customized checklists of care and clinical decision support driven by point-of-care data entry into an electronic health registry. The public health system of Palestine is currently implementing a national electronic registry (eRegistry) for maternal and child health. This trial is embedded in the national implementation and aims to assess the effectiveness of the eRegistry's interactive checklists and clinical decision support, compared with the existing paper based records, on improving the quality of care for pregnant women.
Methods: This two-arm cluster randomized controlled trial is conducted in the West Bank, Palestine, and includes 120 clusters (primary healthcare clinics) with an average annual enrollment of 60 pregnancies. The intervention tool is the eRegistry's interactive checklists and clinical decision support implemented within the District Health Information System 2 (DHIS2) Tracker software, developed and customized for the Palestinian context. The primary outcomes reflect the processes of essential interventions, namely timely and appropriate screening and management of: 1) anemia in pregnancy; 2) hypertension in pregnancy; 3) abnormal fetal growth; 4) and diabetes mellitus in pregnancy. The composite primary health outcome encompasses five conditions representing risk for the mother or baby that could have been detected or prevented by high-quality antenatal care: moderate or severe anemia at admission for labor; severe hypertension at admission for labor; malpresentation at delivery undetected during pregnancy; small for gestational age baby at delivery undetected during pregnancy; and large for gestational age baby at delivery. Primary analysis at the individual level taking the design effect of the clustering into account will be performed as intention-to-treat.
Discussion: This trial, embedded in the national implementation of the eRegistry in Palestine, allows the assessment of process and health outcomes in a large-scale pragmatic setting. Findings will inform the use of interactive checklists and clinical decision support driven by point-of-care data entry into an eRegistry as a health systems-strengthening approach.
Trial registration: ISRCTN trial registration number, ISRCTN18008445 . Registered on 6 April 2017.
Keywords: Antenatal care; Clinical decision support; Electronic registry; Health surveillance; Health systems; Interactive checklists; Maternal and newborn health; Quality of care; eHealth; eRegistries.
Conflict of interest statement
Ethics approval and consent to participate
The study will be conducted according to the Palestinian MoH regulations and PNIPH research policies and procedures. All PHC under study, intervention and control alike, have been notified by the MoH in Palestine about the research. Women are not required to give informed consent to be part of the national health information system (the eRegistry). This trial is considered nonmedical health systems research and uses anonymous health data. Individuals are not regarded as research participants as all treatment decisions remain the responsibility of the healthcare professionals, and are not determined by the trial allocation [32]. The study protocol has been reviewed by Regional Committee for Health Research Ethics, Section South East B, Norway, and has been cleared for implementation (2016/264 B). The ethics committee in Palestine, the Palestinian Health Research Council, also approved the protocol (PHRC/HC/04/14).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
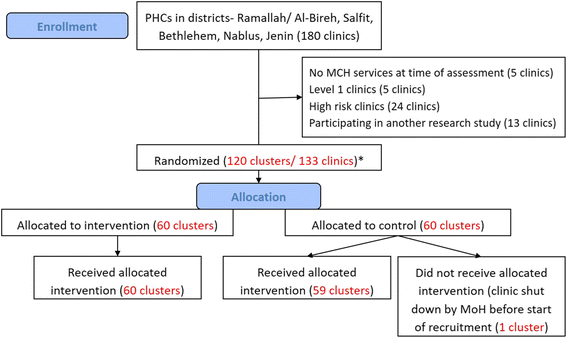

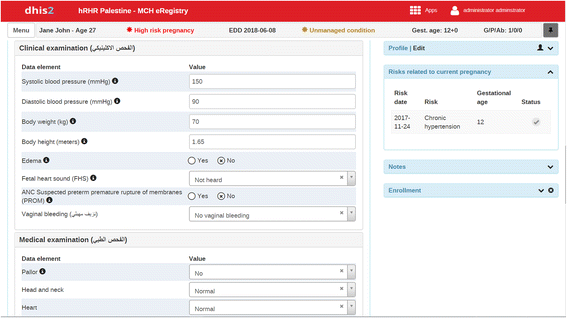


References
-
- World Health Organization. What is universal health coverage? http://www.who.int/health_financing/universal_coverage_definition/en/. Accessed 12 Dec 2016.
-
- Temmerman M, Khosla R, Bhutta ZA, Bustreo F. Towards a new Global Strategy for Women’s, Children’s and Adolescents’ Health. BMJ: British Medical Journal. 2015;351:h4414. - PubMed
-
- Main EK, Bingham D. Quality improvement in maternity care: promising approaches from the medical and public health perspectives. Curr Opin Obstet Gynecol. 2008;20(6):574–80. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

