Variation in natural exposure to anopheles mosquitoes and its effects on malaria transmission
- PMID: 29357976
- PMCID: PMC5780040
- DOI: 10.7554/eLife.32625
Variation in natural exposure to anopheles mosquitoes and its effects on malaria transmission
Abstract
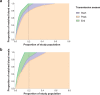
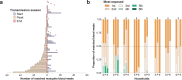
Variation in biting frequency by Anopheles mosquitoes can explain some of the heterogeneity in malaria transmission in endemic areas. In this study in Burkina Faso, we assessed natural exposure to mosquitoes by matching the genotype of blood meals from 1066 mosquitoes with blood from residents of local households. We observed that the distribution of mosquito bites exceeded the Pareto rule (20/80) in two of the three surveys performed (20/85, 76, and 96) and, at its most pronounced, is estimated to have profound epidemiological consequences, inflating the basic reproduction number of malaria by 8-fold. The distribution of bites from sporozoite-positive mosquitoes followed a similar pattern, with a small number of individuals within households receiving multiple potentially infectious bites over the period of a few days. Together, our findings indicate that heterogeneity in mosquito exposure contributes considerably to heterogeneity in infection risk and suggest significant variation in malaria transmission potential.
Keywords: p. falciparum; anopheles; epidemiology; global health; heterogeneity; transmission.
© 2017, Guelbéogo et al.
Conflict of interest statement
WG, BG, LG, JB, SS, JH, KL, SZ, NS, IS, DW, LY, NS, TB, CD No competing interests declared
Figures






References
-
- Bejon P, Williams TN, Nyundo C, Hay SI, Benz D, Gething PW, Otiende M, Peshu J, Bashraheil M, Greenhouse B, Bousema T, Bauni E, Marsh K, Smith DL, Borrmann S. A micro-epidemiological analysis of febrile malaria in Coastal Kenya showing hotspots within hotspots. eLife. 2014;3:e02130. doi: 10.7554/eLife.02130. - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

