Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia
- PMID: 29358182
- PMCID: PMC6027091
- DOI: 10.1182/blood-2017-08-798322
Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia
Erratum in
-
Gökbuget N, Dombret H, Bonifacio M, et al. Blinatumomab for minimal residual disease in adults with B-cell precursor acute lymphoblastic leukemia. Blood. 2018;131(14):1522-1531.Blood. 2019 Jun 13;133(24):2625. doi: 10.1182/blood.2019001109. Blood. 2019. PMID: 31196880 Free PMC article. No abstract available.
Abstract
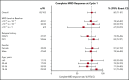
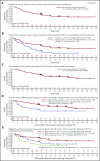
Approximately 30% to 50% of adults with acute lymphoblastic leukemia (ALL) in hematologic complete remission after multiagent therapy exhibit minimal residual disease (MRD) by reverse transcriptase-polymerase chain reaction or flow cytometry. MRD is the strongest predictor of relapse in ALL. In this open-label, single-arm study, adults with B-cell precursor ALL in hematologic complete remission with MRD (≥10-3) received blinatumomab 15 µg/m2 per day by continuous IV infusion for up to 4 cycles. Patients could undergo allogeneic hematopoietic stem-cell transplantation any time after cycle 1. The primary end point was complete MRD response status after 1 cycle of blinatumomab. One hundred sixteen patients received blinatumomab. Eighty-eight (78%) of 113 evaluable patients achieved a complete MRD response. In the subgroup of 110 patients with Ph-negative ALL in hematologic remission, the Kaplan-Meier estimate of relapse-free survival (RFS) at 18 months was 54%. Median overall survival (OS) was 36.5 months. In landmark analyses, complete MRD responders had longer RFS (23.6 vs 5.7 months; P = .002) and OS (38.9 vs 12.5 months; P = .002) compared with MRD nonresponders. Adverse events were consistent with previous studies of blinatumomab. Twelve (10%) and 3 patients (3%) had grade 3 or 4 neurologic events, respectively. Four patients (3%) had cytokine release syndrome grade 1, n = 2; grade 3, n = 2), all during cycle 1. After treatment with blinatumomab in a population of patients with MRD-positive B-cell precursor ALL, a majority achieved a complete MRD response, which was associated with significantly longer RFS and OS compared with MRD nonresponders. This study is registered at www.clinicaltrials.gov as #NCT01207388.
© 2018 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: R.C.B. is an advisor for Amgen, Novartis, AstraZeneca, GenMab, GeMoab, and Pfizer and reports patent royalties from Amgen. J.E.B. is a former Amgen employee and stockholder. M. Bonifacio reports consulting fees from Amgen, Pfizer, Bristol-Myers Squibb, and Ariad Pharmaceuticals (Incyte) and research funding from Novartis. M. Brüggemann reports consulting fees from Amgen, Incyte, and Roche and research funding from Affimed and Regeneron. H. Dombret is an advisor for, serves on a speakers’ bureau for, and reports research support, consultancy, honoraria, and travel/accommodation support from Amgen; is an advisor for and reports research support and honoraria from Roche/Genentech; is an advisor for, serves on a speakers’ bureau for, and reports honoraria and travel/accommodation support from Pfizer; is an advisor for, serves on a speakers’ bureau for, and reports research support, honoraria, and travel/accommodation support from Ariad (Incyte); is an advisor for and reports research support and honoraria from Jazz Pharma and Kite Pharma; is an advisor for and reports honoraria from Novartis, Agios, Sunesis, Ambit (Daiichi Sankyo), Karyopharm, Menarini, Astellas, Janssen, Servier, Seattle Genetics, and Cellectis; and is a consultant and advisor for, serves on a speakers’ bureau for, and reports honoraria from Celgene. C.F. serves on an advisory board for and reports research support associated with the present work from Amgen. N.G. serves on an advisory board and speakers’ bureau for and reports research support associated with the present work and travel support from Amgen and serves on an advisory board and speakers’ bureau for and reports travel support from Pfizer. V. Haddad is a former Amgen employee and stockholder. H.-A.H. reports research support associated with the present work from Amgen. D.N. is an employee and stockholder of and reports patent royalties from Amgen. J.S. is an Amgen employee and stockholder. M.S.T. serves on an advisory board for and reports travel support from Amgen, reports travel support from Roche, serves on an advisory board for and reports travel support from Affimed and Regeneron, and serves on an advisory board for Gilead and Jazz Pharma. H.W. is a former Amgen employee and former stockholder. G.Z. is an employee and stockholder of and reports patent royalties from Amgen. The remaining authors declare no competing financial interests.
Figures
Comment in
-
Blinatumomab for MRD+ B-ALL: the evidence strengthens.Blood. 2018 Apr 5;131(14):1497-1498. doi: 10.1182/blood-2018-02-830364. Blood. 2018. PMID: 29622532 No abstract available.
References
-
- van der Velden VH, Hochhaus A, Cazzaniga G, Szczepanski T, Gabert J, van Dongen JJ. Detection of minimal residual disease in hematologic malignancies by real-time quantitative PCR: principles, approaches, and laboratory aspects. Leukemia. 2003;17(6):1013-1034. - PubMed
-
- Bassan R, Spinelli O, Oldani E, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood. 2009;113(18):4153-4162. - PubMed
-
- Brüggemann M, Raff T, Flohr T, et al. ; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107(3):1116-1123. - PubMed
-
- Gökbuget N, Kneba M, Raff T, et al. ; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120(9):1868-1876. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

