Population Pharmacokinetics of High-Dose Tigecycline in Patients with Sepsis or Septic Shock
- PMID: 29358291
- PMCID: PMC5913959
- DOI: 10.1128/AAC.02273-17
Population Pharmacokinetics of High-Dose Tigecycline in Patients with Sepsis or Septic Shock
Abstract
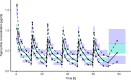
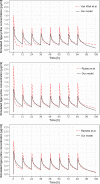
Tigecycline is a glycylcycline often used in critically ill patients as the antibiotic of last resort. The pharmacokinetics (PK) of tigecycline in intensive care unit (ICU) patients can be affected by severe pathophysiological changes so that standard dosing might not be adequate. The aim of this study was to describe population PK of high-dose tigecycline in patients with sepsis or septic shock and evaluate the relationship between individual PK parameters and patient covariates. The study population consisted of 37 adult ICU patients receiving a 200-mg loading dose of tigecycline followed by multiple doses of 100 mg every 12 h. Blood samples were collected at 0.5, 2, 4, 8, and 12 h after dose administration. A two-compartment model with interindividual (IIV) and interoccasion (IOV) variability in PK parameters was used to describe the concentration-time course of tigecycline. The estimated values of mean population PK parameters were 22.1 liters/h and 69.4 liters/h for elimination and intercompartmental clearance, respectively, and 162 liters and 87.9 liters for volume of the central and peripheral compartment, respectively. The IIV and IOV in clearance were less than 20%. The estimated values of distribution volumes were different from previously published values, which might be due to pathophysiological changes in ICU patients. No systematic relationship between individual PK parameters and patient covariates was found. The developed model does not show evidence that individual tigecycline dosing adjustment based on patient covariates is necessary to obtain the same target concentration in patients with sepsis or septic shock. Dosing adjustments should be based on the pathogens, their susceptibility, and PK targets.
Keywords: pharmacokinetics; population pharmacokinetics.
Copyright © 2018 Borsuk-De Moor et al.
Figures




References
-
- Wyeth Pharmaceuticals, Inc. 2016. Tygacil package insert. Wyeth Pharmaceuticals, Inc., Philadelphia, PA.
-
- Hoffmann M, DeMaio W, Jordan RA, Talaat R, Harper D, Speth J, Scatina J. 2007. Metabolism, excretion, and pharmacokinetics of [14C] tigecycline, a first-in-class glycylcycline antibiotic, after intravenous infusion to healthy male subjects. Drug Metab Dispos 35:1543–1553. doi:10.1124/dmd.107.015735. - DOI - PubMed
-
- Food and Drug Administration. FDA drug safety communication: FDA warns of increased risk of death with IV antibacterial Tygacil (tigecycline) and approves new boxed warning. U.S. Food and Drug Administration, Silver Spring, MD.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

