6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio) hexanol: a promising new anticancer compound
- PMID: 29358310
- PMCID: PMC5809612
- DOI: 10.1042/BSR20171440
6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio) hexanol: a promising new anticancer compound
Abstract
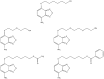
The 7-nitro-2,1,3-nitrobenzoxadiazole (NBD) derivatives are a series of compounds containing the NBD scaffold that are not glutathione (GSH) peptidomimetics, and result in a strong inhibition of glutathione S-transferases (GSTs). Growing evidences highlight their pivotal roles and outstanding anticancer activity in different tumor models. In particular, 6-(7-nitro-2,1,3-benzoxadiazol-4-ylthio) hexanol (NBDHEX) is extensively studied, which is a very efficient inhibitor of GSTP1-1. It triggers apoptosis in several tumor cell lines and this cytotoxic activity is observed at micro and submicromolar concentrations. Importantly, studies have shown that NBDHEX acts as an anticancer drug by inhibiting GSTs catalytic activity, avoiding inconvenience of the inhibitor extrusion from the cell by specific pumps and disrupting the interaction between the GSTP1-1 and key signaling effectors. Additionally, some researchers also have discovered that NBDHEX can act as late-phase autophagy inhibitor, which opens new opportunities to fully exploit its therapeutic potential. In this review, we summarize the advantages, anticancer mechanisms, and analogs of this compound, which will establish the basis on the usage of NBDHEX in clinical applications in future.
Keywords: GSTP1-1 (Glutathione S-transferase Pi); JNK (c-Jun N-Terminal Kinase); NBDHEX (6- (7-nitro-2,1,3-benzoxadiazol-4-ylthio) hexanol); anticancer.
© 2018 The Author(s).
Conflict of interest statement
The authors declare that there are no competing interests associated with the manuscript.
Figures

References
-
- Tew K.D., et al. (1996) Glutathione-associated enzymes in the human cell lines of the National Cancer Institute Drug Screening Program. Mol. Pharmacol. 50, 149–159 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

