Ras-Mediated Activation of the Raf Family Kinases
- PMID: 29358316
- PMCID: PMC6311149
- DOI: 10.1101/cshperspect.a033746
Ras-Mediated Activation of the Raf Family Kinases
Abstract
The extracellular signal-regulated kinase (ERK) cascade comprised of the Raf, MEK, and ERK protein kinases constitutes a key effector cascade used by the Ras GTPases to relay signals regulating cell growth, survival, proliferation, and differentiation. Of the ERK cascade components, the regulation of the Raf kinases is by far the most complex, involving changes in subcellular localization, protein and lipid interactions, as well as alterations in the Raf phosphorylation state. The Raf kinases interact directly with active, membrane-localized Ras, and this interaction is often the first step in the Raf activation process, which ultimately results in ERK activation and the downstream phosphorylation of cellular targets that will specify a particular biological response. Here, we will examine our current understanding of how Ras promotes Raf activation, focusing on the molecular mechanisms that contribute to the Raf activation/inactivation cycle.
Copyright © 2019 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures


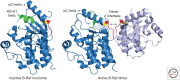

References
-
- Abraham D, Podar K, Pacher M, Kubicek M, Welzel N, Hemmings BA, Dilworth SM, Mischak H, Kolch W, Baccarini M. 2000. Raf-1-associated protein phosphatase 2A as a positive regulator of kinase activation. J Biol Chem 275: 22300–22304. - PubMed
-
- Brennan DF, Dar AC, Hertz NT, Chao WC, Burlingame AL, Shokat KM, Barford D. 2011. A Raf-induced allosteric transition of KSR stimulates phosphorylation of MEK. Nature 472: 366–369. - PubMed
-
- Brummer T, Naegele H, Reth M, Misawa Y. 2003. Identification of novel ERK-mediated feedback phosphorylation sites at the C-terminus of B-Raf. Oncogene 22: 8823–8834. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
